COVID-19: Cardiac Arrhythmias
| Take Home Messages |
|---|
|
Introduction
The novel Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus mainly affects the pulmonary tissue as a primary source of infection, and respiratory symptoms are the most frequent cause of hospital presentations.1 Cardiac complications including arrhythmias are common in COVID-19 and are associated with increased morbidity and mortality.2-5 Consequently, there has been a growing interest in trying to understand the underlying pathophysiological mechanisms. In this editorial, we will outline the arrhythmias reported in COVID-19 to date and discuss some of the underlying mechanisms and management.
Arrhythmias reported in COVID-19
The available data on the prevalence of arrhythmias in COVID-19 is limited and comprises of observational studies, case series or case reports of hospitalised patients (see Table 1).4-13At present, there is a large observational study aiming to recruit 10 000 participants to further categorise cardiac arrhythmias in patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04358029).
In one study, palpitations were reported in 7.3% of patients who were admitted to a hospital with COVID-19 in the Hubei province, China.2 Cardiac arrhythmias were reported in nearly 17% of the hospitalised patients who were tested positive in a single centre in Wuhan city. This figure increased to almost 44% in those who were admitted to the intensive care units.5
In a case series from a single centre in China, malignant ventricular arrhythmias were reported in 11.5% and 5.2% of the hospitalised patients with elevated and normal cardiac troponin respectively,11 whereas in another case series of 393 patients in New York City, nearly 7.5% of patients had atrial tachyarrhythmias.10 In the latter study, atrial arrhythmias were more prevalent in mechanically ventilated patients than non-mechanically ventilated patients (17.7% versus 1.9% respectively). In another single centre observational study in China, pre-existing atrial fibrillation was reported in 3.6% of hospitalised patients with COVID-19.8
Other tachyarrhythmias described in case reports of patients with COVID-19 include atrial fibrillation,9 12 atrial flutter,9 supraventricular tachycardia,13 monomorphic ventricular tachycardia7 and polymorphic ventricular tachycardia (the same case was described in two independent similarly timed publications).12 13 Transient complete heart block has also been reported in a patient with critical COVID-19 leading to cardiac arrest, with the recovery of atrioventricular conduction post resuscitation.6
Sinus tachycardia is common in patients with COVID-19 and likely to represent a physiological response to illness. Importantly, in patients with SARS-CoV-1, persistent sinus tachycardia was commonly observed following discharge and convalescence, suggesting the possibility of an abnormal pathophysiological reaction extending beyond the normal physiological response to the viral infection.14 However, a similar finding has not been yet reported in SARS-CoV-2.
Table 1. Arrhythmias reported in COVID-19 hospitalisations 4-13 | ||||
|---|---|---|---|---|
Author / Country | Study type | Number of patients | Arrhythmia reported | Details |
Mehra et al.4 Internationalb | Observational study MC | 8910 | Not specified | 304 patients (3.4%) had cardiac arrhythmias. Cardiac arrhythmias were more common among non-survivors (6.8%) than survivors (3.2%) of COVID-19. Arrhythmias were independently associated with increased mortality risk (OR 1.95, 95% CI 1.33-2.86). |
Goyal et al.10 USA | Observational study MC | 393 | Not specified | 29 patients (7.5%) had cardiac arrhythmias. Atrial arrhythmias were more common in invasively ventilated patients (17.7%) than non-invasively ventilated patients (1.9%). |
Wang et al. 5 China | Observational study SC | 138 | Not specified | 23 patients (16.7%) had arrhythmia. Arrhythmias were more common in ICU patients (44.4%) than non-ICU patients (6.9%). |
Guo et al.11 China | Case series SC | 187 | VT/VF | 11 patients (5.9%) had VT/VF. VT/VF were more common in patients with elevated troponin (11.5%) v normal troponin (5.2%). |
Deng et al.8 China | Observational study SC | 112 | AF | 4 patients (3.6%) had pre-existing AF. |
Kochav et al.12 USA | Case series SC | 2 | CHB | Transient CHB presented on day 6 of illness (acute CHB happened whilst the patient was intubated on day 6 of illness with PEA arrest). |
Azarkish et al.6 Iran | Case report | 1 | CHB | Transient CHB occurred on day 14 of hospitalisation (one-day post-intubation). |
Seecheran et al.13 Trinidad | Case report | 1 | AF, AFL | The patient presented with AFL. DCCV reverted the rhythm to AF, then it was chemically cardioverted to SR with Amiodarone. |
Kochav et al 12 USA | Case report | 1 | AF | AF occurred on day 7, whilst the patient was intubated requiring DCCV. |
Fried et al 9 USA | Case report | 1 | SVT | The patient developed SVT (>200bpm) following intubation on day 7 of illness requiring DCCV. |
Fried et al. 9 USA Kochav et al.12 USA a | Case report | 1 | VT | Polymorphic VT occurred due to azithromycin induced QTc prolongation requiring DCCV after intubation and inotropes. This happened following presentation with acutely decompensated heart failure and severely reduced LV systolic function. |
Beri et al.7 USA | Case report | 1 | VT | The patient presented with monomorphic VT on day 3 of illness causing cardiac arrest. |
a The same case was published by two independent authors. b 169 hospitals in 11 countries in Asia, Europe, and North America. AF atrial fibrillation, AFL atrial flutter, CI confidence interval, DCCV direct current cardioversion, ICU intensive care unit, LV left ventricular, MC multicentre, PEA pulseless electrical activity, OR odds ratio, QTc heart-rate corrected QT interval, SC single centre, SR sinus rhythm, SVT supraventricular tachycardia, VF ventricular fibrillation, VT ventricular tachycardia. | ||||
Suggested mechanisms of cardiac arrhythmia in COVID-19
The underlying mechanisms of cardiac arrhythmia in patients with COVID-19 are yet to be fully understood but are thought to be multifactorial1 15 16 (see Figure 1):
(i) Cardiac injury due to direct viral infection or indirect hypoxia in the presence of severe acute respiratory infection is thought to be the leading cause;
(ii) Over-activation of the immune system causes a surge in the release of multiple inflammatory cytokines/chemokines with associated myocyte degeneration, conduction abnormalities and/or autonomic dysfunction;1 16
(iii) QTc-interval prolongation due to medication side effects and drug interactions may provoke arrhythmias (e.g. torsades de pointes). Several of the concomitantly used drugs such as macrolides or fluoroquinolones can potentially alter drug metabolism by inhibiting CYP450 enzyme in the liver and induce further QTc-interval prolongation. QTc interval prolongation may also occur due to ischaemia, sympathetic activation, inflammation,17 and congenital long QT syndrome;
(iv) Precipitated by other co-pathologies including systemic hypotension, coronary plaque rupture, pulmonary embolism, electrolyte abnormalities, the unmasking of Brugada syndrome due to fever;18
(v) Arrhythmias independent of COVID-19 may be seen coincidentally (e.g. pre-existing undiagnosed AF).8
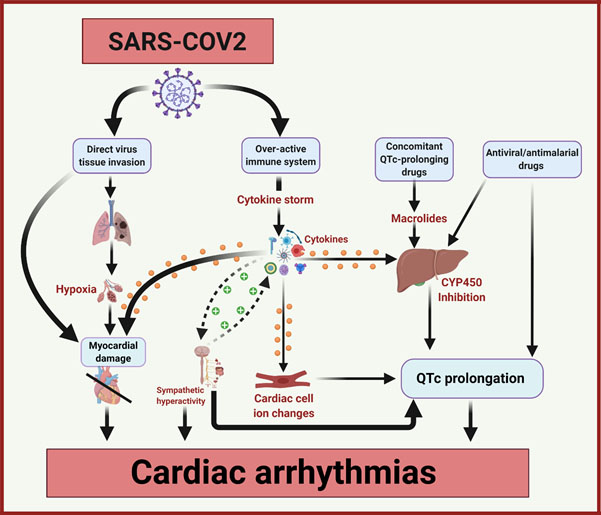
Figure 1. The mechanisms of cardiac arrhythmias in COVID-19. Adapted from Lazzerini et al.16
COVID-19 coronavirus disease-2019, CYP450 cytochrome P450 enzymes, QTc heart rate corrected QT interval, SARS-COV-2 severe acute respiratory syndrome-coronavirus-2.
Acute myocardial injury
Acute myocardial injury is believed to be the leading cause of enhanced cardiac arrhythmias in patients with COVID-19. It is defined as an elevation of high sensitivity cardiac troponin (hs-cTn) above the 99th percentile of its upper limit of normal, and it has a variable incidence rate of approximately 8-12% amongst patients with COVID-19.19 Irrespective of the actual incidence rate, high levels of hs-cTn are strongly correlated with disease severity and higher mortality rates.3 5 20
Proposed causes for acute cardiac injury with SARS-CoV-2 include direct virus inoculation, delayed systemic inflammation, myocardial oxygen demand-supply mismatch, acute coronary events, and iatrogenic causes. Direct (i.e. non-coronary) myocardial damage and severe systemic inflammation appear to be the most serious aetiologies.15 They are of vital clinical significance due to their direct association with fulminant myocarditis progressing to cardiogenic shock.21 For further details about cardiomyocyte injury, the reader could refer to the previous editorial (COVID-19: Implications for Cardiologists).22
Over-activation of the immune system (cytokine storm)
The immune response following SARS-CoV-2 infection can be subdivided into three phases23 (see Figure 2):
- Early infectious;
- Intermediate pulmonary;
- Late severe hyperinflammatory phase.
In clinical practice, there is significant overlap among these phases during the time course of the disease.20 While the majority of patients (81%) are asymptomatic or only mildly affected, a small proportion have a critical illness requiring intensive care support.
Phase I. Early infectious
In the first phase, there is direct viral inoculation of the lung parenchyma and an early innate immune response (e.g. macrophages and monocytes). The clinical course is usually mild and typically includes constitutional symptoms. There is also evidence that SARS-CoV-2 may enter the myocardium, causing inflammation and direct myocardial injury.24 Angiotensin-converting enzyme-2 (ACE2) receptors found within the myocardium and are thought to be a port of entry for the SARS-CoV-2 virus into myocardial cells. In animal studies, adverse myocardial remodelling and myocardial dysfunction were found to be associated with the disintegration of ACE2 receptors, and that was associated with increased risk of cardiac arrhythmias in SARS- CoV-1.11 24-26
Phase II. Intermediate pulmonary
The second phase is hallmarked by hypoxemia due to acute lung injury and subsequent oxygen demand-supply mismatch with myocardial injury. During this phase there is often a switch from aerobic to anaerobic metabolism, resulting in metabolic derangements (e.g. increased intracellular acidaemia, intracellular calcium levels and increased extracellular potassium levels) that can precipitate arrhythmias.16
Phase III. Late severe hyperinflammatory
The third phase occurs in severe cases and is thought to be due an over activation of the host immune system resulting in deleterious effects on distant organs, including the heart resulting in cardiac arrhythmias. This phenomenon is termed as ‘’cytokine syndrome’’ or “cytokine storm” and was well documented in the previous SARS-CoV-1 outbreak in 2003 and with the H1N1 influenza epidemic.26 Cytokine syndrome/storm is a life-threatening condition associated with severe systematic inflammation and multiorgan failure hallmarked by uncontrolled activation of both adaptive (e.g. lymphocytes) and innate (e.g. macrophages) immune responses with the release of inflammatory cytokines including interleukin (IL)-1, 1L-2, IL-6, tumour necrosis factor-alpha and interferon-gamma (see Figure 1). During this phase, there is a release of cytokines/chemokines, which correlate with disease severity and degree of myocardial injury.1 23 The precise mechanisms of myocardial injury and arrhythmia in this phase are not fully understood but may be direct (e.g. effect of cytokines on myocardial cells), or indirect (e.g. myocardial ischaemia, autonomic nervous system dysfunction,17 QTc prolongation27).
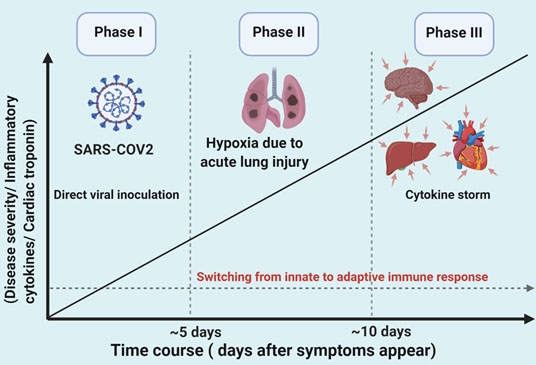
Figure 2. The three phases of SARS-CoV-2 infection (adapted from Akhmerov et al23)
QTc prolongation
Since the disease surfaced in China during late 2019, various classes of medications have been used in an attempt to treat and effectively counter viral replication or curb the inflammatory response generated by the SARS-CoV-2 virus. These include antimalarial, anti-retroviral, monoclonal antibodies and blood plasma products.28 29
Several of the proposed medications for COVID-19 have been associated with cardiac arrhythmias including QTc prolongation, torsades de pointes, atrioventricular block, and sudden cardiac death (see Table 2).23-25 29 30 A large, multicenter, randomized, open-label, placebo-controlled trial is currently ongoing to assess the efficacy and safety of several medications in the treatment of COVID-19; the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial (ClinicalTrials.gov Identifier: NCT04381936) will compare lopinavir/ritonavir, low dose dexamethasone, hydroxychloroquine, azithromycin, and tocilizumab versus no additional treatments. The study is designed such that new or promising treatments may be tested once available. For example, convalescent plasma therapy has recently been included in the trial as a promising therapy for COVID-19.
Table 2. Arrhythmias associated with COVID-19 medications | ||
|---|---|---|
Drugs | Class | Arrhythmia risk |
Remedesivir | Antiviral | Severe hypotension and cardiac arrest31 |
Favipiravir | Antiviral | QTc-interval prolongation in an Ebola-infected patient32 |
Lopinavir/Ritonavir | Anti-retroviral | AV block, PR, QRS, and QTc-interval prolongations33 34 |
Hydroxychloroquine | Antimalarial, immunosuppressant | QTc prolongation, TdP, polymorphic VT/VF35 |
Azithromycin | Macrolide antibiotic | QTc prolongation, TdP, polymorphic VT/VF36 |
AV atrioventricular, QTc heart rate corrected QT interval, TdP torsades de pointes, VF ventricular fibrillation, VT ventricular tachycardia. | ||
Remdesivir, a nucleotide analogue ribose nucleic acid (RNA) polymerase inhibitor, is currently being evaluated for its efficacy in the management of SARS-CoV-2 virus. Although significant cardiac side effects for this drug are yet to be reported, in the PALM trial evaluating its effectiveness in the treatment of Ebola virus one patient in the remdesivir group developed hypotension after the initial loading dose followed by a cardiac arrest.31 Favipiravir (nucleoside analogue RNA polymerase inhibitor) is also being evaluated in patients with moderate COVID-19 disease and has been reported to cause QTc interval prolongation in an Ebola-infected patient.32 The QTc interval returned to normal after discontinuation of the drug. Lopinavir/Ritonavir associated atrioventricular block and prolongation of PR interval and QRS duration has been reported when used concomitantly with Atazanavir.34 However, the incidence of these conduction abnormalities appears infrequent. In a study published by Cao et al., assessing the efficacy of Lopinavir/Ritonavir in patients with severe COVID-19, only 1 out of the 95 patients developed QTc interval prolongation. No other electrical rhythm adverse effects were reported in this study.33
Clinical trials of tocilizumab, a humanized IL-6 receptor antibody (commonly used in the treatment of rheumatoid arthritis and other inflammatory arthropathies) are ongoing to assess its efficacy in SARS-CoV-2 pneumonia (ClinicalTrials.gov Identifier: NCT04317092) Accumulating evidence suggests that inflammatory cytokines may have a direct effect on the QTc interval.37-41 IL-6 inhibition, commonly used in the treatment of chronic inflammatory conditions has been shown to reduce QTc interval in patients with rheumatoid arthritis.38 The pro-Inflammatory cytokines TNF-alpha and IL-1 have been shown to prolong the cardiomyocyte action potential, through HERG K channels39 and Ca channels,40 respectively. Elevations in serum IL-6, TNF-alpha and IL-1 have been observed in patients with rheumatoid arthritis presenting with QTc prolongation and Torsades de Pointes.41 These studies raise the possibility that IL-6 inhibition may reduce the arrhythmic burden in patients with COVID-19 and cytokine storm.
Hydroxychloroquine, chloroquine, and macrolides are all associated with QTc-prolongation, torsade de pointes and polymorphic VT.35 36 These medications are discussed in a previous editorial (Use of Hydroxychloroquine and Azithromycin in treatment of COVID-19 and its relation to QTc prolongation).42
Management of cardiac arrhythmias in COVID-19
The management of cardiac arrhythmias in COVID-19 is similar to the management of arrhythmias in patients without COVID-19; however, there are a few additional considerations which are summarised in Table 3.43-48 The restructuring of cardiac arrhythmia services due to the pandemic are discussed in a previous editorial (COVID-19: impact on cardiology procedural services).49 Further guidance has recently been published by the European Society of Cardiology for the diagnosis and management of cardiovascular disease in COVID-19.43
Table 3. Additional considerations for management of cardiac arrhythmias in COVID-19 |
|---|
|
a Further advice is provided by the Association of Inherited Cardiac Conditions (AICC).48 AVB atrioventricular block, DOAC direct oral anticoagulant, ICD implantable cardioverter defibrillators, INR international normalised ratio, PPE personal protective equipment, PPM permanent pacemaker, QTc heart rate corrected QT interval, TdP torsades de pointes, UK United Kingdom, VF ventricular fibrillation, VT ventricular tachycardia. |
Conclusion
Cardiac arrhythmias are a common complication of COVID-19. It is associated with increased morbidity and mortality risk in hospitalised patients. The mechanisms of arrhythmia in COVID-19 are not fully understood but are commonly due to cardiac injury, overactivation of the immune system and drug-induced QTc prolongation. The management of cardiac arrhythmias in COVID patients is mostly similar to non-COVID patients; however, there are a few essential considerations including more rigorous ECG monitoring when using QTc prolonging medications, deferral of permanent pacemaker implantation in high-grade AV block and conservative approach to acute atrial arrhythmias (e.g. rate control rather than rhythm control). Further studies are ongoing and will help improve our understanding of the incidence, prevalence, and mechanisms of cardiac arrhythmias in COVID-19.
Disclosures
None.
References
- Kuck KH. Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz 2020 doi: 10.1007/s00059-020-04924-0
- Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025-31. doi: 10.1097/CM9. 0000000000000744
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054-62. doi: 10.1016/S0140-6736(20)30566-3
- Mehra MR, Desai SS, Kuy S, et al. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med 2020 doi: 10.1056/NEJMoa2007621
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020 doi: 10.1001/jama. 2020.1585
- Azarkish M, Laleh Far V, Eslami M, et al. Transient complete heart block in a patient with critical COVID-19. Eur Heart J 2020 doi: 10.1093/eurheartj/ehaa307
- Beri A, Kotak K. Cardiac injury, Arrhythmia and Sudden death in a COVID-19 patient. HeartRhythm Case Rep 2020 doi: 10.1016/j.hrcr.2020.05.001
- Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020 doi: 10.1016/j.ijcard.2020.03.087
- Fried JA, Ramasubbu K, Bhatt R, et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020 doi: 10.1161/CIRCULATIONAHA.120.047164
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med 2020 doi: 10.1056/ NEJMc2010419
- Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020 doi: 10.1001/jamacardio. 2020.1017
- Kochav SM, Coromilas E, Nalbandian A, et al. Cardiac Arrhythmias in COVID-19 Infection. Circ Arrhythm Electrophysiol 2020 doi: 10.1161/CIRCEP.120.008719
- Seecheran R, Narayansingh R, Giddings S, et al. Atrial Arrhythmias in a Patient Presenting With Coronavirus Disease-2019 (COVID-19) Infection. J Investig Med High Impact Case Rep 2020;8:2324709620925571. doi: 10.1177/232470962092 5571
- Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006;82(964):140-4. doi: 10.1136/pgmj.2005.037515
- Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr 2020;14(3):247-50. doi: 10.1016/j.dsx.2020. 03.013
- Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, Arrhythmic Risk and Inflammation: Mind the Gap! Circulation 2020 doi: 10.1161/CIRCULATIONAHA.120.047293
- Adlan A. Sympathetic nervous system dysfunction in rheumatoid arthritis: brief overview 2019 [Available from: http://www.brainimmune.com/sympathetic-nervous-system-dysfunction-rheumatoid-arthritis/ accessed 30/05/2020.
- Chang D, Saleh M, Garcia-Bengo Y, et al. COVID-19 Infection Unmasking Brugada Syndrome. HeartRhythm Case Rep 2020 doi: 10.1016/j.hrcr.2020.03.012
- Mehra MR, Ruschitzka F. COVID-19 Illness and Heart Failure: A Missing Link? JACC Heart Fail 2020 doi: 10.1016/j.jchf.2020.03.004
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5
- Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020 doi: 10.1093/eurheartj/ehaa190
- Sharrack N. COVID-19: Implications for Cardiologists 2020 [Available from: https://www.britishcardiovascularsociety.org/ resources/editorials/articles/covid-19-implications-cardiologists accessed 30/05/2020.
- Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res 2020;126(10):1443-55. doi: 10.1161/CIRCRESAHA.120.317055
- Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39(7):618-25. doi: 10.1111/j.1365-2362.2009.02153.x
- Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417(6891):822-8. doi: 10.1038/ nature00786
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004;25(6):291-4. doi: 10.1016/j.tips.2004.04.001
- Adlan AM. Inflammation and Heart Rate-corrected QT Interval: Evidence for a Potentially Reversible Cause of Sudden Death in Patients with Rheumatoid Arthritis? J Rheumatol 2018;45(12):1609-10. doi: 10.3899/jrheum.180921
- Abd El-Aziz TM, Stockand JD. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect Genet Evol 2020;83:104327. doi: 10.1016/j.meegid.2020.104327
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020;19(3):149-50. doi: 10.1038/d41573-020-00016-0
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006;12(10):1203-7. doi: 10.1038/nm1477
- Mulangu S, Dodd LE, Davey RT, Jr., et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med 2019;381(24):2293-303. doi: 10.1056/NEJMoa1910993
- Chinello P, Petrosillo N, Pittalis S, et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis 2017;11(12):e0006034. doi: 10.1371/journal.pntd.0006034
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020;382(19):1787-99. doi: 10.1056/NEJMoa2001282
- Rathbun CR, Liedtke MD, Blevins SM, et al. Electrocardiogram abnormalities with atazanavir and lopinavir/ritonavir. HIV Clin Trials 2009;10(5):328-36. doi: 10.1310/hct1005-328
- Kapoor A, Pandurangi U, Arora V, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: A scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J 2020;20(3):117-20. doi: 10.1016/j.ipej.2020.04.003
- Choi Y, Lim HS, Chung D, et al. Risk Evaluation of Azithromycin-Induced QT Prolongation in Real-World Practice. Biomed Res Int 2018;2018:1574806. doi: 10.1155/2018/ 1574806
- Adlan AM, Panoulas VF, Smith JP, et al. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J Rheumatol 2015;42(3):421-8. doi: 10.3899/jrheum.140861
- Lazzerini PE, Acampa M, Capecchi PL, et al. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 2015;67(3):332-9. doi: 10.1002/acr.22455
- Wang J, Wang H, Zhang Y, et al. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem 2004;279(14):13289-92. doi: 10.1074/jbc.C400025200
- Li YH, Rozanski GJ. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc Res 1993;27(3):525-30. doi: 10.1093/cvr/27.3.525
- Lazzerini PE, Capecchi PL, Bertolozzi I, et al. Marked QTc Prolongation and Torsades de pointes in Patients with Chronic Inflammatory Arthritis. Front Cardiovasc Med 2016;3:31. doi: 10.3389/fcvm.2016.00031
- Tindale AH, S. Use of Hydroxychloroquine and Azithromycin for treatment of COVID-19 and its relation to QTc prolongation 2020 [Available from: https://www. britishcardiovascularsociety.org/resources/ editorials/articles/hydroxychloroquine-azithromycin-treatment-covid-19-qtc-prolongation accessed 30/05/2020.]
- European Society of Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic 2020 [Available from: https://www.escardio.org/ Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance accessed 30/05/2020.]
- British Society of Echocardiography. Clinical guidance regarding provision of echocardiography during the COVID-19 pandemic 2020 [Available from: https://bsecho.org/covid19 accessed 30/05/20.]
- NHS England and NHS Improvement. Clinical guide for the management of cardiology patients during the coronavirus pandemic 2020 [Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-cardiolgy-coronavirus-v1-20-march.pdf accessed 30/05/2020.]
- Resuscitation Council UK. Resuscitation Council UK Statement on COVID-19 in relation to CPR and resuscitation in acute hospital settings 2020 [Available from: https://www. resus.org.uk/media/statements/resuscitation-council-uk-statements-on-covid-19-coronavirus-cpr-and-resuscitation/covid-healthcare/ accessed 30/05/2020.]
- Wise J. Covid-19 and thrombosis: what do we know about the risks and treatment? BMJ 2020;369:m2058. doi: 10.1136/bmj.m2058 [published Online First: 2020/05/23]
- Association for Inherited Cardiac Conditions. COVID-19 2020 [Available from: https://theaicc.org/?page_id=649 accessed 30/05/2020.]
- Kurdi H. COVID-19: Impact on cardiology procedural services 2020 [Available from: https://www. britishcardiovascularsociety.org/resources/editorials/articles/covid-19-impact-cardiology-procedural-services accessed 30/05/2020.]
Community Events Calendar


