Use of Hydroxychloroquine and Azithromycin for treatment of COVID-19 and its relation to QTc prolongation
Introduction
As the novel coronavirus SARS-CoV-2, known widely as COVID-19, continues to spread globally, the search for pharmaceutical treatments has intensified. Given the speed at which the pandemic is progressing, much focus has fallen on repurposing drugs with known antiviral effects. Therefore, attention has fallen on hydroxychloroquine and azithromycin as potential drug therapies for COVID-19. Due to the pace at which the situation is changing, there has been a deluge of evidence of varying quality publicised in academic, mainstream and social media streams. This editorial will thus look at the rationale for using these drugs in the treatment of COVID-19, the evidence that has accumulated so far, and the cardiovascular safety profile of using these drugs with recommendations on how to mitigate their risks.
Rationale
Chloroquine and Hydroxychloroquine (HCQ) have been used for many years as antimalarials. More recently, their immunomodulatory properties have been used specifically for treatment of auto-immune disorders such as Systemic Lupus Erythematosis (SLE). Furthermore, chloroquine has been shown to have significant in-vitro effects on viral replication in a variety of viruses including HIV, Influenza A, and most pertinently the 2003 SARS Coronavirus where it showed a 99% reduction in viral replication1-3. Building on these studies, Liu et al. (2020) recently showed that the less toxic hydroxychloroquine was also effective in vitro against COVID-194. Therefore, in both China and France, regulatory bodies have allowed the use of hydroxychloroquine.
Similarly, azithromycin has long been studied for its use as an anti-inflammatory and anti-viral in addition to its roles as an antibacterial. It is the only antibiotic known to significantly reduce exacerbations of COPD5, around half of which are caused by viral, rather than bacterial infections. Mechanistically, it seems likely that it exerts its effect via upregulation of interferons and interferon-stimulated genes6.
In Vivo Evidence
Against this background, there has been a move to trialling hydroxychloroquine in patients with COVID-19. Although there are at least 10 clinical trials planned in China, there is only one small randomised trial of 30 patients answering this question at present published after peer review and a further study from Wuhan province of 62 patients that has been published “preprint” and is yet to be peer-reviewed. Furthermore, there is an 80-patient observational study and a 36-patient non-randomised trial from the same group in France.
The published randomised controlled trial was conducted in Shanghai and randomised 15 patients in each arm to standard care (including antivirals) or standard care plus 400mg daily of hydroxychloroquine7. The primary outcome measure was viral PCR-negative throat swabs at day 7. This endpoint was met in 14/15 patients in the standard care group and 13/15 patients in the hydroxychloroquine group. There was also no difference in other markers of disease progression such as radiological progression, and no deaths in the study. Therefore, based upon this small trial, there is no evidence for hydroxychloroquine alone in treating COVID-19.
The second, yet-to-be peer reviewed paper from Wuhan did show some benefit of hydroxychloroquine on COVID-19 pneumonia progression using CT progression as an endpoint8. This trial randomised 31 patients to each arm (control vs treatment) with both arms including standard of care including antivirals and antibiotics with occasional steroid use. The treatment regime added hydroxychloroquine 200mg BD. The patients in the treatment group appeared to be slightly more unwell on average with higher rates of cough and fever. Despite this, 81% of the patients in the hydroxychroloquine group had improved radiological features of pneumonia on CT compared to 55% of the control group. All 4 patients that progressed to severe disease were in the control group. These results are promising but it must be noted that this paper has not yet been peer-reviewed.
The data from France is interesting but cannot be viewed as practice-changing. The non-randomised trial followed 42 COVID-19 positive patients in Marseilles9. All patients were offered hydroxychloroquine – those that turned it down or had exclusion criteria were therefore used as controls. This left 26 patients in the hydroxychloroquine group and 16 controls, although 6 patients in the treatment group were lost to follow-up. Furthermore, of the 20 patients analysed in the control group, six had azithromycin 500mg stat and 250mg OD thereafter. The endpoint used for analysis was negative viral PCR in nasal swabs at day 6. On this primary endpoint, there was a reduction in positive viral PCR swabs at day six: 70% of the treatment group were negative compared to 12.5% of the control group.
There are at least four major problems with this data for recommending use of Azithromycin and hydroxychloroquine clinically:
- The sample size is small
- The non-randomised nature of the trial introduced bias and only patients with exclusion criteria, that included comorbidities, were placed in the control arm. The baseline characteristics between the groups were different
- 6 patients out of 26 in the treatment group were lost to follow-up, of which one died and 3 were transferred to ICU due to deterioration, favouring the treatment regimen
- The endpoint is a surrogate endpoint, and was changed post-trial – the initial endpoints are listed in the submitted protocol to the EU trials database as detection rates at days 1,4,7 and 14
Based on the results of this trial, the same group released an observational study of 80 patients in the same institution treated with hydroxychloroquine and azithromycin10. Again, the endpoint was PCR-negativity in throat swabs at various time-points. Although the results appeared favourable compared to other published data from an unwell patient population in China (case fatality rate 1.2% in this study vs 28% in the comparator study), the differences in the patient populations are too great to make any statements. However, the combination of azithromycin and hydroxychloroquine was tolerated well, had a good safety profile in these 80 patients, and certainly warrants further study in the form of a randomised controlled trial.
Cardiac Safety Profile
A major concern about using hydroxychloroquine +/- azithromycin is the potential for cardiac side-effects, in particular lengthening the QT interval and increasing the incidence of sudden cardiac death. Hydroxychloroquine increases the QT interval by blocking the KCNH2-encoded hERG/Kv11.1 potassium channel11. There have been 222 documented cases of QT prolongation and its sequelae secondary to hydroxychloroquine in the FDA’s adverse drug reaction reporting system, and 105 cardiac arrests11. There have been several case reports detailing Torsades de Pointes due to hydroxychloroquine – these have generally occurred with chronic hydroxychloroquine usage and often in the context of a pre-existing borderline prolonged QTc. It must also be noted that the half-life of hydroxychloroquine is long, in the range of 32-50 days12. This explains the long QT syndrome is largely in chronic use as the drug accumulates, but is also reassuring if the expected length of use for treating COVID-19 will be measurable in days or weeks.
Azithromycin is also associated with lengthening of the QT interval. Guinea-pig models suggest this could be due to inhibition of L-type calcium and sodium channels13. The effect seems to be relatively modest and most pronounced in patients with other risk factors for lengthening of the QT, and is largely based upon observational data14. Furthermore, of those patients with long QT syndrome secondary to azithromycin, only 1% of these will develop ventricular arrhythmias15. Looking at patients with baseline normal QTc intervals from a randomised trial for azithromycin vs amoxicillin in preventing COPD exacerbations, only 1 out of 1142 patients randomised to Azithromycin 250mg BD developed QTc prolongation5, compared to 2 patients in the amoxicillin group. Therefore, the risk of QTc prolongation with azithromycin is present, but very modest in low-risk populations.
An important benefit of azithromycin compared to other macrolides must be noted; although macrolides in general inhibit cytochrome p450, azithromycin is the standalone compound in this class with a 15-member, rather than 14-member lactone ring. The result of this is a reduction in inhibition of cytochrome enzymes, which reduces the direct interactions with other drugs16.
Finally, the combination of hydroxychloroquine and azithromycin has not been properly assessed from a safety perspective, although it seems plausible that there is an additive effect on QTc prolongation. Old animal data has shown no increase in action potential alternans of azithromycin in addition to chloroquine compared to chloroquine alone17. The small datasets from the Marseilles hydroxychloroquine and azithromycin trials have shown no adverse effects, but high-risk patients were excluded from receiving this combination of treatments and only 80 patients were enrolled9.
Risk Stratification for QTc Prolongation
As a result of this and other data, Giudicessi et al’s11 excellent paper proposes a “traffic light” system for assessing the risk of QT prolongation in patients being treated with hydroxychloroquine and azithromycin. The patients are divided into three categories: green, orange or red.
Using baseline data from the Mayo institute’s 1400 patients with congenital long QTc, they propose a cut-off of 470ms for men and 480ms for women. All patients should receive an ECG at baseline and then again at 48 and 96 hours.
“Green” patients: All men with a QTc below 470ms and women <480ms should be considered low risk and can receive azithromycin and hydroxychloroquine provided that the QTc does not lengthen by either >60ms compared to baseline or to >500 ms.
“Orange” patients have a QTc between 470/480ms and 500ms. This should prompt a pause for thought and a correction of underlying electrolyte disturbances / discontinuation of other QTc-prolonging therapies, but should not limit drug administration.
“Red” patients have a QTc > 500ms at baseline and should have ECG monitoring at baseline, 2 hours, 48 hours and 96 hours if therapy is deemed critical. Clearly, at present the evidence for hydroxychloroquine and azithromycin is weak, and hence administration of only one agent at a time if necessary, with potential telemetry monitoring, may be advisable.
An alternative approach to risk stratification could be to document the “Risk Score for Drug-Associated QTc Prolongation”18.

Table 1: Risk Score Calculation
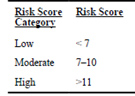
Table 2: Risk Score categorisation
This score could potentially be used in a similar way to the “green” “orange” and “red” scoring system above, with high risk patients requiring closer monitoring and a stronger indication for hydroxychloroquine and/or azithromycin treatment than those in lower-risk groups.
Clinical Use
There is a desperate clinical need for therapeutic options in this current COVID-19 crisis and this is reflected with the FDA granting Emergency Use Authorisation for hydroxychloroquine and chloroquine to treat COVID-19. In contrast the European Medicines Agency have said that COVID-19 patients can receive the drugs as part of clinical trials or through national emergency use programs. Ultimately the onus is on the physician to make a decision on whether the evidence for safety and efficacy outweighs the risks of using these drugs. Indeed, based on the studies presented many clinicians worldwide are widely using these drugs because of the lack of alternative options.
Summary
- Theoretical and in vitro studies suggest that hydroxychloroquine and azithromycin may be effective in reducing viral replication in COVID-19 infection.
- In vivo evidence of efficacy is lacking at this present time with only one small published randomised controlled trial performed, with no evidence of benefit, although an as-yet un-peer reviewed trial does show a reduction in disease severity from hydroxychloroquine in addition to standard care.
- Non-randomised studies have shown the potential for a reduction in viral load but should be treated as anecdotal at this time.
- Both hydroxychloroquine and azithromycin have the potential to prolong the QT interval and provoke cardiac arrhythmias – however the risk is low if patients have a normal baseline QTc interval and no other risk factors including pre-existing conduction disease or other QT-prolonging drugs.
- There is insufficient evidence to support use of either drug outside of clinical trials.
Recommendations
- Hydroxychloroquine and azithromycin can be considered for use in COVID-19 patients outside of a clinical trial setting in consultation with local infectious disease/virology services.
- Ideally duration of treatment should be short-term (5-10 days) to cover the acute phase of illness.
- If hydroxychloroquine and azithromycin are started:
- should be in conjunction with cardiology to monitor QT interval
- patients should be risk stratified on the basis of their baseline QTc intervals using gender specific cut-offs of 470ms for men and 480ms for women
- baseline serum potassium and magnesium, renal and hepatic function must be assessed
- if available connect patient to telemetry
- stop and avoid ALL OTHER non-essential QT prolonging drugs
- monitor ECG at regular interval depending upon their risk profile
- monitor and optimise serum potassium and magnesium daily
- if there is any doubt regarding the measurement of the QTc please seek an experienced cardiologist to measure the QTc interval.
References
- Romanelli F., Smith K.M., Hoven A.D., 2004. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr Pharm Des. 10(21): 2643-8.
- Vincent M.J., Bergeron E., Benjannet S., et al., 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005. 2: 69
- Ooi EE, Chew JS, Loh JP, Chua RC., 2006. In vitro inhibition of human influenza A virus replication by chloroquine. Virol J. 3: 39.
- Liu, J., Cao, R., Xu, M., Wang, X., Zhang, H., Hu, H., Li, Y., Hu, Z., Zhong, W. and Wang, M., 2020. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery, 6(1), pp.1-4.
- Albert R.K., Connett J., Bailey W.C., Casaburi R., Cooper J.A. Jr, Criner G.J., Curtis J.L., Dransfield M.T., Han M.K., Lazarus S.C., Make B., Marchetti N., Martinez F.J., Madinger N.E., McEvoy C., Niewoehner D.E., Porsasz J., Price C.S., Reilly J., Scanlon P.D., Sciurba F.C., Scharf S.M., Washko G.R., Woodruff P.G., Anthonisen N.R; COPD Clinical Research Network, 2011. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. Aug 25;365(8):689-98. doi: 10.1056/NEJMoa1104623.
- Gielen V., Johnston S.L., Edwards M.R., 2010. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. Sep;36(3):646-54.
- Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., 2020. ,A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) Journal of Zhejiang University, March 2020 (epub)
- Chen, Z., Hu, J., Zhang, Z., Jiang, S., Han, S., Yan, D., Zhang, R., Hu, B., Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomised clinical trial. Pre-print before publication. https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v2
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D., 2020. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20:105949.
- Gautret P., Lagier J-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P., Hocquart M., Finance J., Esteves Vieira V., Tissot Dupont H., Honoré S., Stein A., Million M., Colson P, La Scola B., Veit V., Jacquier A., Deharo J-C., Drancourt M., Fournier P.E, Rolain J-M., Brouqui P, Raoult D., 2020. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study https://www.mediterranee-infection.com/wp-content/uploads/2020/03/COVID-IHU-2-1.pdf
- Giudicessi J.R., Noseworthy P.A., Friedman P.A., and Ackerman M.J., 2020. Urgent Guidance for Navigating and Circumventing the QTc Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for COVID-19. Mayo Clinic Proceedings. Pre-pub https://mayoclinicproceedings.org/pb/assets/raw/Health%20Advance/journals/jmcp/jmcp_covid19.pdf
- Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M., 2018. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. Oct;41(10):919-931.
- Zhang M., Xie M., Li S., Gao Y(., Xue S., Huang H., Chen K., Liu F., Chen L., 2017. Electrophysiologic Studies on the Risks and Potential Mechanism Underlying the Proarrhythmic Nature of Azithromycin. Cardiovasc Toxicol. Oct;17(4):434-440. doi: 10.1007/s12012-017-9401-7.
- Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M., 2012. Azithromycin and the risk of cardiovascular death. N Engl J Med. May 17;366(20):1881-90.
- Sears S.P., Getz T.W., Austin C.O., Palmer W.C., Boyd E.A., Stancampiano F.F., 2016. Incidence of Sustained Ventricular Tachycardia in Patients with Prolonged QTc After the Administration of Azithromycin: A Retrospective Study. Drugs Real World Outcomes. Mar;3(1):99-105.
- Sharma, K.K., Sangraula, H., Das, B.P., Badyal, D.K. and Dadhich, A.P., 2002. Cytochrome P450 and drug interactions. Indian journal of pharmacology, 34(5), p.289.
- Fossa A.A., Wisialowski T., Duncan J.N., Deng S., Dunne M., 2007. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg. Nov;77(5):929-38.
- Tisdale, J. E., Jaynes, H. A., Kingery, J. R., Mourad, N. A., Trujillo, T. N., Overholser, B. R., & Kovacs, R. J. (2013). Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circulation. Cardiovascular quality and outcomes, 6(4), 479–487
