What does the new decade hold for ventricular tachycardia ablation?
| Take Home Messages |
|---|
|
Introduction
Ventricular arrhythmias (VA) manifest in a variety of forms ranging from benign single premature ventricular complexes (PVCs) to potentially life-threatening sustained ventricular tachycardia (VT) and ventricular fibrillation (VF). There are multiple therapeutic options available in the management of ventricular arrhythmias including anti-arrhythmic drugs, implantable cardioverter defibrillator (ICD) therapy and catheter ablation (see Figure 1).
There has been exponential progress in the field of catheter ablation of VA over the last two decades. As the field of electrophysiology rapidly expands and the number of VA ablations worldwide continues to increase, multiple national cardiac electrophysiology societies across the globe have collaborated to produce an expert consensus statement on catheter ablation of VA in 20191 as an update and supplement to the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death2 and the 2015 European Society of Cardiology (ESC) Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death.3 In this article I will briefly outline the evidence and developments in VT ablation focusing on ischaemic VT.
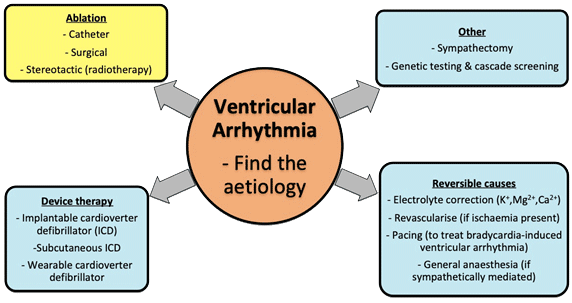
Figure 1. Broad overview of the management of ventricular arrhythmia
VT mechanisms and aetiology
The general approach in the management of VT depends on the aetiology, in other words, whether or not the heart is structurally abnormal (i.e. ischaemic or non-ischaemic cardiomyopathy) or normal (commonly outflow tract in origin). This takes into account the three fundamental mechanisms of a tachyarrhythmia: re-entry (the main mechanism in scar-related VT), abnormal automaticity and triggered activity.4 Table 1 explains some of the terms used in defining the different ventricular arrhythmia.
Table 1. Definitions of the different types of ventricular arrhythmias | |
|---|---|
Terms | Explanation |
PVC/PVB/VE | A premature beat arising from an ectopic focus within the ventricle. PVCs may be unifocal or multifocal and can manifest as single isolated beats or in repeating patterns: i.e. bigeminy (every alternate beat is a PVC), trigeminy (every third beat is a PVC), quadrigeminy (every fourth beat is a PVC), couplet (2 consecutive PVCs). The origin of each PVC can be discerned from the 12 lead ECG QRS morphology: LBBB morphology (dominant S wave in V1) implies RV origin while RBBB morphology (dominant R wave in V1) implies LV origin. |
NSVT | A minimum of 3 consecutive ventricular beats lasting up to 30 seconds with a heart rate of more than 100 beats/minute. |
Sustained VT | A tachycardia (more than 100 beats/minute) originating from the ventricle, which lasts for more than 30 seconds or less than 30 seconds if termination is required due to haemodynamic compromise.5 |
Slow VT | There are infrequent occasions where VT can manifest with a heart rate of less than 100 beats/minute. Slow VT is usually encountered in patients taking anti-arrhythmic drugs or those with a large area of ventricular scar (i.e. large VT macro-reentry circuit). |
Monomorphic VT | A broad complex tachycardia with the same ECG QRS morphology. In patients with structural heart disease monomorphic VT is commonly a result of scar macro-reentry tachycardia, whilst in structurally normal hearts it is commonly due to automaticity from a focal region (e.g. outflow tract tachycardia). In patients with ischaemic heart disease, monomorphic VT is usually scar-mediated and typically occurs a few years after index myocardial infarction. |
Polymorphic VT | Polymorphic VT refers to tachycardia whereby the ECG QRS morphology varies from beat to beat and is commonly due to acute and reversible pathology (e.g. ischaemia, electrolyte abnormality and digitalis toxicity), but rarely due to inherited cardiac conditions (e.g. catecholaminergic polymorphic VT – a rare arrhythmogenic disorder that is adrenergic-induced6). |
Torsade de Pointes | A specific form of polymorphic VT (“twisting” of the QRS complexes around the isoelectric line) associated with QT prolongation. TdP can be due to congenital (i.e. long QT syndrome) or acquired QT prolongation (i.e. drug induced, electrolyte imbalance, ischaemia). |
Bidirectional VT | A rare type of polymorphic VT characterised by beat-to-beat alternation of the QRS axis (either left to right axis or LBBB to RBBB). This is most commonly associated with digoxin toxicity and is a characteristic feature in catecholaminergic polymorphic VT. |
ECG electrocardiogram, LBBB left bundle branch block, LV left ventricle, NSVT non-sustained ventricular tachycardia, PVC premature ventricular complexes, RBBB right bundle branch block, RV right ventricle, TdP Torsade de Pointes, VE ventricular ectopic, VPB ventricular premature beats, VT ventricular tachycardia. | |
VT catheter ablation indications
Patients with structural heart disease who experience VT have an elevated risk for mortality and sudden cardiac death and a secondary prevention implantable cardioverter-defibrillator (ICD) has been shown to reduce mortality by 28% (driven by a 50% reduction in arrhythmic death).7 ICDs terminate VT by using anti-tachycardia pacing (delivering a few seconds of pacing stimuli at a rate faster than the VT to pace-terminate the arrhythmia) or delivery of a direct current (DC) shock. Although ICDs effectively terminate VT (up to 38% receive an appropriate shock for VA within 5 years8), it does not prevent VT. Unsurprisingly, recurrent VT and repetitive ICD shocks can have a significantly negative impact on morbidity and mortality.9,10 Anti-arrhythmic drugs (e.g. Amiodarone) can be used to prevent VT recurrence however Amiodarone use has been associated with undesirable toxicity and increased mortality risk.11 Invasive catheter ablation is the only treatment available to modify the arrhythmogenic substrate in VT and has been shown to reduce risk of VT recurrence as well as delay VT recurrence in randomised controlled trials.1–3,12,13,14 Consequently, guidelines recommend the use of catheter ablation as an adjunctive therapy to prevent VT recurrence and repetitive ICD therapies.1–3 Data from the United Kingdom,15 Europe16 and the United States of America17 has shown an exponential growth in VT ablations in line with increased ICD implantation rates
In contrast, VT or PVCs in the setting of a structurally normal heart carries a lower risk for sudden cardiac death and an ICD is infrequently indicated (i.e. haemodynamically unstable VT).3 In this group of patients, catheter ablation is potentially curative and can be considered for either symptomatic relief or to treat tachycardia-associated cardiomyopathy.18
Randomised controlled trials on catheter ablation of VT
Improved patient selection criteria and catheter ablation techniques
Table 2 describes a brief overview of the history of VT ablation. Up until the last two decades, there were no randomised clinical trials of catheter ablation of VT due to multiple challenges. Some of the challenges include the inability to blind the patient to VT ablation to obtain a control group, high cross-over (and drop out) rates between VT ablation and anti-arrhythmic drug therapy groups, heterogeneity of mapping and ablation techniques, catheters and equipment, rapid technological development which then make the findings of any long-term trial less clinically relevant, lack of consensus on short and long-term success and late presentation of patients who are usually very unwell.19
Table 2. Brief overview of the history of VT ablation | |
|---|---|
Period | Significant historical event (s) |
1959 | The first reported case of a successful non-pharmacological treatment of VT came from Couch in North America.20 The patient was a 57-year-old lady with ischaemic cardiomyopathy who had episodes of VT that was difficult to treat despite quinidine. Following excision of her ventricular aneurysm, her VT resolved despite the cessation of quinidine. |
Early to mid 1970s | Left ventricular aneurysmectomy was routinely performed for pre-operative VT patients with previous myocardial infarction despite high mortality rates (18%) and VT recurrence rates (50% during the post-operative hospitalisation period).21 |
Late 1970s to early 1980s | Removing just the endocardium from arrhythmogenic tissue with epicardial sparing (endocardial encircling ventriculotomy) was introduced by Guiraudon et al but was associated with marked post-operative LV dysfunction likely due to interruption of the coronary arterial supply.22 Guiraudon et al also attempted to isolate the RV free wall in arrhythmogenic RV cardiomyopathy but this was associated with progressive RV failure.23Josephson et al helped fine tune the precision of surgical endocardial resection by developing electrical activation mapping techniques to delineate the border zone between scar and healthy tissue.24Adjunctive cryoablation (exposing tissue to extremely cold temperature to cause discrete, homogenous lesions) was utilised in areas not easily resected or an isthmus (a channel of slow conduction bounded by 2 lateral lines of functional block due to surviving myocardium located between scarred areas). Overall, the success rates of surgery for VT was 90% but at the expense of a 5-15% mortality rate.25 |
1983 | Catheter ablation of VT endocardially was first described by Hartzler and energy was delivered via DC electrical shock.26 In one of the largest early studies (n=43) by Fontaine et al, the success rates in preventing VT recurrence were 56% (not requiring anti-arrhythmic drugs) and 44% (requiring anti-arrhythmic drugs) over a mean follow-up of 29±12 months. Four deaths were related to the procedure but no deaths were thought to be related to the endocardial shock itself.27 |
Late 1980s | Concerns regarding the complications of high energy discharge with DC energy ablation such as impaired left ventricular function, cardiac rupture, barotrauma and the need for general anaesthesia led to the development and increased use of radiofrequency (RF) energy.28,29 RF ablation utilises a sinusoidal high-frequency (500-750Hz) current that creates discrete lesions via thermal injury.30 Compared to DC energy, RF ablation could be performed on conscious patients. As a result, RF energy for catheter ablation of VT replaced the surgical approach as the treatment of choice for refractory VT. |
1990s | RF energy replaced DC energy as the energy delivery of choice.31 The use of RF energy was accompanied by rapid developments in VT mapping (entrainment, substrate, pace, activation) and ablation technologies. |
2000 to 2020 | The last two decades heralded several multicenter randomised control trials in catheter ablation of VT in ischaemic cardiomyopathy patients, which are described in the next section. |
DC direct current, RF radiofrequency, RV right ventricle, VT ventricular tachycardia. | |
A number of randomised controlled trials have been undertaken on catheter ablation of VT on patients with structural heart disease; mainly ischaemic cardiomyopathy (see Table 3). These studies have allowed refinement of patient selection criteria, optimal timing and ablation techniques which if applied in real world settings can translate into efficacy and safety comparable to RCTs.32,33 Traditionally, VT ablation was only reserved for patients with incessant VT (drug and shock refractory) usually occurring in end-stage heart failure process and associated with poor outcomes. These studies support the use of catheter ablation earlier in the disease (e.g. following ICD therapy or even as primary prophylaxis) where outcomes are favourable. Furthermore, traditional ablation techniques relied heavily on activation mapping (e.g. determining the optimal ablation site whilst the patient was in VT). Such an approach would not be feasible in haemodynamically unstable VT and consequently success rates were poor. More recent developments have demonstrated high success rates using a substrate modification approach which can be performed during sinus or paced rhythm. For example in one RCT, using a substrate modification approach during catheter ablation for VT resulted in 91% freedom from ICD shocks after 2 years with a complication rate of 4.7%.
Table 3. Randomised controlled trials for catheter ablation of VT | |||
|---|---|---|---|
Year | Study | Details | Main Outcomes |
2007 | SMASH-VT12 | N=128. Compared catheter ablation of VT post-ICD vs no ablation in ICD patients with previous MI. | Reduction in ICD therapy and VT storm in the ablation group but no significant difference in mortality. |
2010 | VTACH13 | N=110 Compared prophylactic catheter ablation of VT before ICD implantation vs no ablation in patients with VT and history of MI. | Prophylactic VT ablation led to a prolonged time to recurrence of VT (18.6 months in the ablation group vs 5.9months in the control group). |
2015 | CALYPSO 34 | N=27. Pilot trial to determine the feasibility of a large clinical trial aimed at comparing early catheter ablation vs AAD therapy in ICD patients with IHD. | Unfortunately, this trial had difficulty with recruitment as most patients had already been established on an AAD. |
2016 | VANISH9 | N=259. Landmark trial compared catheter ablation vs escalated AAD therapy in ischaemic cardiomyopathy patients with ICD who had VT despite being on established AAD therapy. | VT ablation led to reduction in the composite primary endpoint of death, VT storm or appropriate ICD shock compared to escalation of AAD. This was driven by a reduction in VT storm and appropriate ICD shock. |
2017 | SMS14 | N=111. Compared prophylactic catheter ablation vs no ablation in ischaemic cardiomyopathy patients with ICDs and unstable ventricular tachyarrhythmias. | VT ablation led to a reduction in the number of ICD therapies but no difference in the primary endpoint (time to first VT/VF recurrence) |
2020 | BERLIN VT35 | N=159. Compared a preventive VT ablation strategy pre-ICD implantation vs the routine practice of deferred ablation strategy after multiple ICD therapies in patients with stable ischaemic cardiomyopathy and documented VT. | No difference in the composite primary endpoint of all-cause mortality and hospitalisation for heart failure or arrhythmia. There was a reduction in sustained VT and appropriate ICD therapy in the preventive ablation group. |
AAD antiarrhythmic drug, BERLIN VT Preventive Ablation of Ventricular Tachycardia in Patients with Myocardial Infarction, CALYPSO Catheter ablation for Ventricular Tachycardia in Patients with an Implantable Cardioverter Defibrillator, ICD implantable cardioverter defibrillator, MI myocardial infarction, SMASH VT Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia, SMS Substrate Modification Study, VANISH Ventricular Tachycardia Ablation versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease, VF ventricular fibrillation, VT ventricular tachycardia, VTACH Substrate Modification in Stable Ventricular Tachycardia in Addition to ICD Therapy. | |||
What does the new decade hold for the future of VT ablation?
There are a number of key developments that we can expect during the coming decade for VT ablation (see Figure 2), in the following three domains:
- Mechanical circulatory support during ablation;
- Novel and ongoing technological developments;
- Alternatives/adjuncts to radiofrequency (RF) ablation.
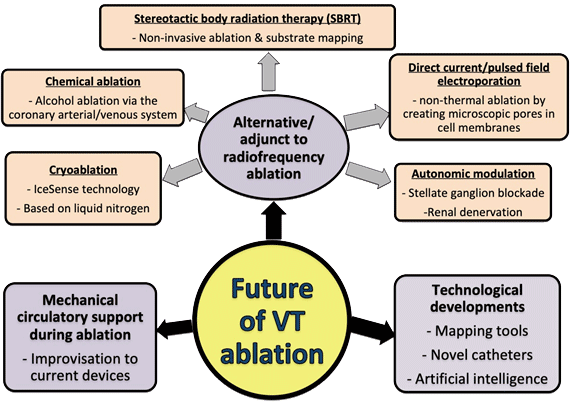
Figure 2. The developments in VT ablation that we can expect during this decade
1. Mechanical circulatory support during ablation
Acute haemodynamic decompensation occurs in 11% of patients undergoing VT ablation and is associated with increased mortality.36 Even if the VT was haemodynamically stable and tolerated, there may be an increased risk of impaired end organ perfusion during VT ablation. Mechanical circulatory support (MCS) devices may facilitate haemodynamic stability and preserve end organ perfusion during sustained VT to allow long periods of mapping.36 Current options available include the intra-aortic balloon pump, TandemHeart left atrial to femoral artery bypass system, Impella left ventricle to aorta flow-assist system and extracorporeal membrane oxygenation (ECMO). The risk of complications from MCS use such as vascular access, bleeding and thromboembolic risk as well as the increased cost precludes its routine use. Therefore, further improvisations and newer MCS devices, risk stratification tools and randomised trials are needed in this decade to ensure that high-risk VT ablation patients obtain the best short and long-term outcome.
2. Novel and ongoing technological developments
This decade will see the electrophysiology community learn more about and potentially expand their adoption of emerging technologies. It is hoped that these technologies will improve mapping and ablation techniques translating into better safety and efficacy outcomes for patients undergoing catheter ablation of VT. For example, high density mapping catheters have improved substrate definition and provide rapid mapping during VT which is particularly useful in patients with haemodynamically unstable VT. The use of contact force catheters and intracardiac echocardiography can help reduce the risk of complications. Much research is ongoing to identify the optimal and safest method of delivering larger and deeper lesions e.g. lower ionic strength irrigants, energy delivery guided by impedance modulation, simultaneous unipolar and bipolar ablation and novel ablation catheters (including retractable needle-tip electrode catheter which can ablate arrhythmogenic substrates that are conventionally difficult to reach e.g. mid wall or septal).37 Advances in artificial intelligence has allowed the development of software to predict VT inducibility as well as guide successful ablation sites.38 The software integrates detailed scar assessment using pre-procedural cardiac magnetic resonance imaging to create a virtual heart. Models using the virtual heart can predict VT circuits and areas of successful ablation. However, these image-based simulation models are yet to be used prospectively in patients.
3. Alternatives/adjuncts to radiofrequency ablation
Several promising alternatives to RF energy delivery for VT ablation have emerged in the last decade and it will be interesting to see how much of the following techniques will be adopted to routine practice in this decade (see Table 4).
Table 4. Alternatives/adjuncts to radiofrequency catheter ablation of VT | |
|---|---|
Technique | Description |
Stereotactic body radiation therapy guided by non-invasive substrate mapping | Cuculich et al have performed this technique on 5 patients with refractory VT and SBRT has been shown to significantly reduce the burden of VT at 46 months.39 With a mean ablation time of only 14 minutes, the most attractive aspect of this technique is that ablation of VT can be performed in a non-invasive catheter-free manner.39 One can imagine that in the not too distant future, a patient with VT can walk into an SBRT machine, receive the treatment and be cured of VT on the very same day. |
Direct current or pulsed field electroporation | This technique ablates in a non-thermal fashion by creating microscopic pores in cell membranes and is already trialled in some centres for atrial fibrillation ablation.40 Could this technique be applied to VT ablation? |
Chemical ablation | Alcohol ablation from the coronary arterial or venous system is a useful technique for ablating areas that are difficult to reach with conventional endocardial (and even epicardial if close to the coronary arteries) ablation.41–43 |
Cryoablation | Surgical cryoablation has been used for more than 3 decades for both cardiac and non-cardiac procedures.44 Catheter cryoablation is used with increasing frequency in AF ablation but the freezing power is limited and is primarily designed for thinner atrial tissue.45 The use of catheter cryoablation for the ventricle has so far only been limited to case reports.46,47 Berte et al have shown in a proof-of-concept study that the IceSense technology (based on liquid nitrogen) was able to create larger and deeper endocardial and epicardial ventricular lesions.48 Further data will be of interest to assess the safety and efficacy of this technology. |
Autonomic modulation | There is an increasing recognition that the autonomic nervous system has an important influence on both atrial and ventricular arrhythmia.49 In terms of the treatment of ventricular arrhythmia, trials are ongoing to evaluate the potential benefits of renal denervation as an adjunct to VT ablation (REDRESS VT (NCT02856373a), RESET-VT (NCT01858194a), RESCUE (NCT01747837a)) and stellate ganglion blockade (NCT02646501a). |
a Accessible from clinicaltrials.gov SBRT Stereotactic body radiation therapy, VT ventricular tachycardia. | |
Ongoing randomised controlled trials on VT ablation
The aforementioned RCTs have enlightened our understanding of the benefits and risks of catheter ablation of VT but plenty of questions remain: What is the optimal timing of catheter ablation of VT? Is there a role for primary prevention VT ablation? Can non-invasive scar assessment help predict patients that are likely to develop VT? More randomised controlled studies are ongoing to address some of these questions and are listed in Table 5.
Table 5. Future randomised controlled trials on catheter ablation of VT | |||
|---|---|---|---|
Trial namea | Patient group | Intervention | Primary outcome |
PARTITA (NCT01547208) | ICM or NICM with first ICD shock | Early VT ablation vs ablation only when electrical storm occurs | Composite of HF hospitalisation & all-cause mortality |
PAUSE-SCD (NCT02848781) | ICM or NICM | ICD followed by either catheter ablation or AAD | Composite of recurrent VT, CV rehospitalisation & all-cause mortality |
IMPRESS (NCT03531502) | ICM or NICM who experience a first ICD shock will undergo NIPS procedure to induce VT via their ICD | If NIPS positive, patients allocated to either VT ablation or AAD | Number of recurrent ICD shocks |
PREVENTIVE VT (NCT03421834) | ICM with CTO of infarct-related area | ICD & VT ablation vs ICD only | Composite of time to first ICD therapy & VT-related hospitalisation |
VANISH2 (NCT02830360) | Prior MI & history of VT/ICD therapy | VT ablation vs AAD (Amiodarone/Sotalol) | All-cause mortality Time to first ICD shock Number of VT storm Time to VT requiring cardioversion or ICD therapy Time to incessant VT |
a Accessible from clinicaltrials.gov AAD anti-arrhythmic drug, CTO chronic total occlusion, CV cardiovascular, HF heart failure, ICD implantable cardioverter defibrillator, ICM ischaemic cardiomyopathy, MI myocardial infarction, NICM non-ischaemic cardiomyopathy, NIPS non-invasive programmed stimulation, VT ventricular tachycardia. | |||
Conclusion
Catheter ablation of VT has come a long way since its first description in 198326 and multiple studies since then have supported its utility in the management of patients with VT. The encouraging results from the recent VANISH trial have paved the way for the recommended use of catheter ablation of VT in ischaemic cardiomyopathy patients with an ICD who present with recurrent monomorphic VT/VT storm/ICD shocks despite AAD therapy.1 With emerging technologies, innovations and refinements to current tools and the increasing number of studies being conducted in this new decade, the field of VT ablation certainly has a promising and bright future.
References
- Cronin EM, Bogun FM, Maury P et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17(1):e2–154.
- Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2018;138(13):e419–20.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Eur Heart J. 2015;36(41):2793–867.
- Edhouse J, Morris F. Broad complex tachycardia — Part I Mechanisms of ventricular arrhythmias Electrocardiographic findings in monomorphic ventricular tachycardia. BMJ Clin Rev. 2002;324(7339):719–22.
- Issa ZF, Miller JM, Zipes DP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease: Third Edition. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease: Third Edition. 2019.
- Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythmia Electrophysiol. 2012;5:1044–52.
- Connolly SJ, Hallstrom AP, Cappato R et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. Eur Heart J. 2000;21:2071–8.
- Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: The ALTITUDE survival study. Circulation. 2010;122:2359–67.
- Sapp JL, Wells GA, Parkash R et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–21.
- Dunbar SB, Dougherty CM, Sears SF et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: A scientific statement from the American Heart Association. Circulation. 2012;126:2146–72.
- Santangeli P, Muser D, Maeda S et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–1559.
- Reddy VY, Reynolds MR, Neuzil P et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65.
- Kuck KH, Schaumann A, Eckardt L et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375:31–40.
- Kuck KH, Tilz RR, Deneke T et al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: Results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythmia Electrophysiol. 2017;10(3):e004422.
- Murgatroyd F, Cunningham D, Cunningham M et al. National Audit of Cardiac Rhythm Management Devices. National Institute for Cardiovascular Outcomes Research. 2017;1–28.
- Camm JA, Nisam S. European utilization of the implantable defibrillator: Has 10 years changed the enigma? Europace. 2010;12:1063–9.
- Palaniswamy C, Kolte D, Harikrishnan P et al. Catheter ablation of postinfarction ventricular tachycardia: Ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm. 2014;11(11):2056–63.
- Dukkipati SR, Choudry S, Koruth JS et al. Catheter Ablation of Ventricular Tachycardia in Structurally Normal Hearts: Indications, Strategies, and Outcomes—Part I. J Am Coll Cardiol. 2017;70(23):2909–23.
- Pokorney SD, Friedman DJ, Calkins H et al. Catheter ablation of ventricular tachycardia: Lessons learned from past clinical trials and implications for future clinical trials. Heart Rhythm. 2016;13(8):1748–54.
- Couch OA. Cardiac aneurysm with ventricular tachycardia and subsequent excision. Circulation. 1959;20:251–3.
- Mason JW, Stinson EB, Winkle RA et al. Relative efficacy of blind left ventricular aneurysm resection for the treatment of recurrent ventricular tachycardia. Am J Cardiol. 1982;49(1):241–8.
- Guiraudon G, Fontaine G, Frank R et al. Encircling Endocardial Ventriculotomy: A New Surgical Treatment for Life-Threatening Ventricular Tachycardias Resistant to Medical Treatment Following Myocardial Infarction. Ann Thorac Surg. 1978;26(5):438–44.
- Guiraudon GM, Klein GJ, Gulamhusein SS et al. Total disconnection of the right ventricular free wall: Surgical treatment of right ventricular tachycardia associated with right ventricular dysplasia. Circulation. 1983;67:463–70.
- Josephson ME, Harken AH, Horowitz LN. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60:1430–9.
- Miller JM, Kienzle MG, Harken AH et al. Subendocardial resection for ventricular tachycardia: Predictors of surgical success. Circulation. 1984;70:624–31.
- Hartzler GO. Electrode catheter ablation of refractory focal ventricular tachycardia. J Am Coll Cardiol. 1983;2:1107–13.
- Fontaine G, Tonet JL, Frank R et al. Clinical experience with fulguration and antiarrhythmic therapy for the treatment of ventricular tachycardia. Long-term follow-up of 43 patients. Chest. 1989;95:785–97.
- Kay GN, Epstein AE, Dailey SM, Plumb VJ. Role of Radiofrequency Ablation in the Management of Supraventricular Arrhythmias: Experience in 760 Consecutive Patients. J Cardiovasc Electrophysiol. 1993;4:371.
- Odell RC, Lev M. Closed chest catheter desiccation of the atrioventricular junction using radiofrequency energy—A new method of catheter ablation. J Am Coll Cardiol. 1987;9:349.
- Joseph JP, Rajappan K. Radiofrequency ablation of cardiac arrhythmias: Past, present and future. QJM. 2012;105(4):303–14.
- Jackman WM, Wang X, Friday KJ et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White Syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605.
- Field ME, Gold MR, Reynolds MR et al. Real-world outcomes of ventricular tachycardia catheter ablation with versus without intracardiac echocardiography. J Cardiovasc Electrophysiol. 2020;31(2):417–22.
- Adlan AM, Arujuna A, Dowd R et al. Long-term follow-up of normal and structural heart ventricular tachycardia catheter ablation: Real-world experience from a UK tertiary centre. Open Heart. 2019;6(2):e000996.
- Al-Khatib SM, Daubert J, Anstrom K et al. Catheter ablation for ventricular tachycardia in patients with an implantable cardioverter defibrillator (CALYPSO) pilot trial. J Cardiovasc Electrophysiol. 2015;26:151–7.
- Willems S, Tilz RR, Steven D et al. Preventive or Deferred Ablation of Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT): A Multicenter Randomized Trial. Circulation. 2020; 141:1057-67.
- Virk SA, Keren A, John RM et al. Mechanical Circulatory Support During Catheter Ablation of Ventricular Tachycardia: Indications and Options. Heart Lung Circ. 2019;28(1):134–45.
- Guandalini G, Liang J, Marchlinski F. Ventricular Tachycardia Ablation: Past, Present, and Future Perspectives. JACC Clin Electrophysiol. 2019;5(12):1363–83.
- Trayanova NA, Pashakhanloo F, Wu KC et al. Imaging-Based Simulations for Predicting Sudden Death and Guiding Ventricular Tachycardia Ablation. Circ Arrhythmia Electrophysiol. 2017;10(7):e004743.
- Cuculich PS, Schill MR, Kashani R et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325–36.
- Wittkampf FHM, van Es R, Neven K. Electroporation and its Relevance for Cardiac Catheter Ablation. JACC Clin Electrophysiol. 2018;4(8):977–86.
- Okishige K, Nakamura R, Yamauchi Y et al. Chemical ablation of ventricular tachycardia arising from the left ventricular summit. Clin Case Reports. 2019;7(11):2036–41.
- Kay GN, Epstein AE, Bubien RS et al. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1992;19:159–68.
- Baher A, Shah DJ, Valderrabano M. Coronary venous ethanol infusion for the treatment of refractory ventricular tachycardia. Hear Rhythm. 2012;9:1637–9.
- Avitall B, Kalinski A. Cryotherapy of cardiac arrhythmia: From basic science to the bedside. Hear Rhythm. 2015;12:2195–203.
- Mugnai G, De Asmundis C, Ciconte G et al. Incidence and characteristics of complications in the setting of second-generation cryoballoon ablation: A large single-center study of 500 consecutive patients. Heart Rhythm. 2015;12:1476–82.
- Rivera S, De La Paz Ricapito M, Espinoza J et al. Cryoablation for Ventricular Arrhythmias Arising from the Papillary Muscles of the Left Ventricle Guided by Intracardiac Echocardiography and Image Integration. JACC Clin Electrophysiol. 2015;1(6):509–16.
- Marai I, Andria N, Gurevitz O. Cryoablation for Ventricular Tachycardia Originating from Anterior Papillary Muscle of Left Ventricle Guided by Intracardiac Echocardiography. Case Reports Cardiol. 2017;2017:9734795.
- Berte B, Sacher F, Wielandts JY et al. A new cryoenergy for ventricular tachycardia ablation: A proof-of-concept study. Europace. 2017;19(8):1401–7.
- Waldron NH, Fudim M, Mathew JP et al. Neuromodulation for the Treatment of Heart Rhythm Disorders. JACC Basic to Transl Sci. 2019;4(4):546–62.
Community Events Calendar


