Making ventricular tachycardia VANISH: timing of catheter ablation in secondary prevention
| Take Home Messages |
|---|
|
Introduction
Ventricular tachycardia (VT) is a potentially life-threatening arrhythmia associated with increased mortality risk in patients with structural heart disease, of which ischaemic heart disease is the commonest cause. Implantable cardioverter defibrillator (ICD) therapy prevents sudden cardiac death and reduces mortality risk in patients with ischaemic VT.1 ICDs can terminate VT by antitachycardia pacing (ATP) or by delivering a direct current shock, however they cannot prevent VT recurrence. Furthermore ICD shocks are associated with increased patient mortality and morbidity (e.g. psychological distress and physical trauma).2,3 The main treatment options to prevent VT recurrence include anti-arrhythmic drugs (AADs) or catheter ablation. For the purposes of this article the term AADs refers to medications with anti-arrhythmic properties excluding conventional betablockers; in the United Kingdom (UK) Amiodarone is typically first line followed by Mexiletine or Sotalol. Prior to the publication of the VANISH (Ventricular Tachycardia Ablation versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease) trial in 2016,2 ischaemic cardiomyopathy patients who received recurrent ICD shocks while on AAD therapy would typically have their drugs (e.g. Amiodarone, Sotalol) escalated in the first instance as it was shown to be more effective than placebo or beta-blockers in randomised controlled trials (RCTs).4–6 Although consensus statements and guidelines recommended the use of catheter ablation when AAD therapy failed to prevent recurrent VT, they were largely based on expert opinion and case series.7,8
Prior randomised studies have demonstrated benefit of VT ablation in patients with ischaemic heart disease undergoing an ICD for a primary prevention indication.9,10 However, it was unclear as to whether VT ablation was superior to AAD escalation in patients with ischaemic heart disease and recurrent VT despite AAD therapy. Furthermore, the optimal timing of catheter ablation in secondary prevention of VT remained unclear. In this article I will describe two recent RCTs addressing the role of VT ablation in secondary prevention: the VANISH trial2 and the Preventive Ablation of Ventricular Tachycardia in Patients with Myocardial Infarction (BERLIN VT) trial.11
The VANISH trial
How does catheter ablation of VT compare with escalated anti-arrhythmic drug therapy?
In the VANISH trial, Sapp et al performed the first RCT to compare catheter ablation of VT with escalated AAD therapy in patients with ischaemic cardiomyopathy and an ICD who had VT despite first-line AAD therapy.2 This was a multicentre collaboration involving 22 tertiary centres in Canada, Europe, the United States and Australia. Patients were included if they had a myocardial infarction (MI) (but not an acute coronary syndrome or a recent ST elevation MI <1month), an ICD implant and an episode of VT while on Amiodarone or another class I or class III AAD within the previous 6 months. Any one of the following were defined as VT episodes: ≥3 VT episodes treated with ATP (≥1 episode was symptomatic); ≥1 appropriate ICD shocks; ≥3 VT episodes within 24 hours; sustained VT below the programmed detection rate of the ICD. The VT episodes had to also be monomorphic with rates of <250bpm.
Patients allocated to the ablation group underwent the procedure within 14 days of randomisation either under conscious sedation or general anaesthesia. Patients in the escalated AAD group were treated with either oral Amiodarone or Amiodarone plus Mexiletine (200mg three times daily) depending on the drug and dose taken at the time of the index arrhythmia. The AAD escalation protocol was as follows:
- For patients already on Amiodarone<300mg once daily (OD): Amiodarone dose was increaseed (loading dose 400mg twice daily (BD) for 2 weeks then 400mg OD for 1 week then maintenance dose of 300mg OD);
- For patients on Amiodarone>300mg OD: Mexiletine 200mg three times daily was added;
- For patients on another AAD: Amiodarone was initiated (loading dose of 400mg BD for 2 weeks, then 400mg OD for 4 weeks then maintenance dose of 200mg OD).
The primary outcome measure was a composite of death or VT storm (≥3 VT within 24 hours) or appropriate ICD shock after 30-days of treatment. The pre-specified secondary outcomes included each of the primary outcome parameters and adverse effects.
The VANISH trial: Outcomes
In the VANISH trial, 259 patients were randomised with 132 patients allocated to the ablation arm and 127 patients allocated to the escalated AAD arm. No patients were lost to follow-up but 5 patients withdrew from the ablation arm and 4 patients withdrew from the escalated AAD arm. The average age was 67-70 years, predominantly male (93%) with an average of 16 years since last MI. Almost all patients were taking betablockers (~95%), and AADs at baseline predominantly included Amiodarone (~65 %) or Sotalol (~35 %).
During a mean follow up of 28 months, the primary outcome occurred in 59.1% of patients in the ablation group and 68.5% in the escalated AAD group (hazard ratio (HR) 0.72, 95% confidence interval (CI), 0.53-0.98; p=0.04). The significantly lower primary outcome rate in the ablation group was driven mainly by reduction in the rates of appropriate ICD shocks (HR 0.77, 95% CI 0.53- 1.14; p=0.19) and VT storm (HR 0.66, 95% CI 0.42-1.05; p=0.08).
Interestingly, if patients were already on Amiodarone at baseline, the benefit in terms of the primary outcome was seen in the catheter ablation group (HR 0.0.55, 95% CI 0.38-0.80; p=0.001). However, if patients were on a non-Amiodarone AAD, then the benefit of catheter ablation was not observed. There was no difference in mortality between the two groups (HR 0.96, 95% CI 0.60-1.53; p=0.86).
In terms of adverse events, the procedural complications in the ablation group included cardiac perforation (n=2, 1.5%), major bleeding (n=3, 2.3%) and vascular injury (n=3, 2.3%) but there were no procedure-related deaths. However, in the escalated AAD group treatment-related adverse events occurred in more patients (39 vs 20, p=0.003) and were more frequent (51 vs 22, p=0.002). The adverse events in the escalated AAD group included: death (2.4%) from pulmonary toxicity (n=2) and liver dysfunction (n=1), pulmonary infiltration (n=2; 1.6%), thyroid dysfunction (n=10, 7.8%), liver dysfunction n=6, 4.7%) and tremor/ataxia (n=6, 4.7%).
The VANISH trial: Discussion
In summary, in ischaemic cardiomyopathy patients with an ICD who experienced VT despite being on AAD, catheter ablation of VT led to a significantly lower rate of the composite primary outcome of death, VT storm or appropriate ICD shock compared to escalated AAD therapy. Such benefits were only observed in patients who were already on amiodarone when the index arrhythmia occurred. Importantly, the VANISH trial demonstrated that catheter ablation of VT was relatively safe (1.5- 2.3% procedural complications with no mortality) compared to escalated AAD therapy (1.6% death with up to 7.8% treatment-related complications).
There were several important limitations of the trial. Firstly, the treatment allocation was unblinded due to the different nature of both interventions. However subjecting patients to a sham procedure comes with its own ethical challenges and may expose patients to significant procedural risks. Secondly, the trial was inadequately powered to assess the effect of the intervention on a hard end point – mortality. Thirdly, this was a multicentre trial with variations in the procedural experience and outcomes. It may be possible that undergoing VT ablation in a more specialised referral centre may have led to better outcomes. Nevertheless, the inclusion of multiple centres makes the findings more generalisable. Lastly, patients enrolled into the VANISH trial had a high disease burden with advanced disease progression where VT ablation was second-line therapy.
Nevertheless this was a high impact clinical trial which demonstrated important and relevant findings that paved the way for the international guideline recommendation for the use of catheter ablation of VT in ischaemic cardiomyopathy patients with an ICD who present with recurrent monomorphic VT/VT storm/ICD shocks despite AAD therapy.12
The first VT ablation trial published in the new decade: the BERLIN VT tr
Does the timing of VT ablation pre/post-ICD implantation matter?
This was the question addressed by the latest trial on catheter ablation of VT which was published in January 2020 by the BERLIN VT investigators.11 The BERLIN VT trial sought to examine if a preventive VT ablation strategy before a secondary prevention ICD implantation can improve outcomes compared to the routine practice of deferred ablation strategy after multiple ICD therapies. The hypothesis was that VT ablation before ICD implantation would be more effective at preventing subsequent ICD therapies, compared to a deferred ablation strategy. It is worth remembering that this study was different to the VTACH trial which compared a preventive VT ablation strategy against no ablation.10 The SMASH-VT trial was also different as it compared VT ablation post-ICD implantation against no ablation.9 Furthermore, other previous studies had only compared VT ablation with non-ablative therapies.2,13
The BERLIN VT study was a multicentre RCT which included 26 clinical centres in Europe. Patients were included if they had a prior MI (older than 4 weeks), a moderately reduced left ventricular ejection fraction (30-50%), and any stable or lifethreatening ventricular arrhythmias requiring an ICD implantation.
Patients were randomly assigned (1:1) to receive catheter ablation for VT either before ICD implantation (‘preventive ablation strategy’) or after ICD shock for VT (‘deferred ablation strategy’). Patients allocated to the ‘preventive ablation strategy’ group had to undergo the procedure within 2 weeks of enrolment and then receive an ICD. In contrast, patients in the ‘deferred ablation strategy’ group underwent the procedure only after the third appropriate ICD shock for VT. It is worth noting that the patients and investigators were not blinded to the treatment assignment.
The primary outcome measure was a composite of all-cause mortality and unplanned hospitalisation (at least one overnight stay) for either symptomatic VT or worsening heart failure. The secondary outcome measures were: Sustained VT or ventricular fibrillation, appropriate and inappropriate ICD therapy, all-cause mortality, cardiovascular mortality, unplanned hospitalisation for any cause and cardiac reasons, change in quality of life from enrolment to the 12-month follow-up.
The BERLIN VT trial: Outcomes
Using the prespecified criteria for interim analyses, the trial was terminated early due to futility. 163 patients were randomised with 77 patients allocated to the preventive ablation arm and 86 patients allocated to the deferred ablation arm. 4 patients (1 in preventive group, 3 in deferred group) dropped out before any study procedure or follow-up and were excluded from the analysis. In the preventive ablation group, 7/76 (9.2%) patients did not undergo ablation; reasons included non-inducible VT (n=3), ablation refusal (n=2), lack of scarred substrate (n=1) and redirection to cardiac surgery (n=1). As for the 90.8% who underwent ablation, the acute ablation success was 76.8% according to noninducibility of VT. In the deferred ablation group, 10/83 (12%) underwent VT ablation at a median of 46 days after enrolment with an acute ablation success of 80% according to non-inducibility of VT.
During a mean follow up of 418 days in the prevention ablation group and 376 days in the deferred ablation group, there was no difference in the primary outcome (HR 1.09, 95% CI 0.62-1.92; p=0.77). Among the secondary outcomes, the proportion of patients with sustained ventricular tachyarrhythmia (39.7% vs 48.2% p=0.050) and appropriate ICD therapy (34.2% vs 47.0% p=0.030) were numerically reduced in the preventive ablation group (no P-value adjustment for multiple testing). The large standard deviations and small patient numbers made it difficult to draw any further conclusions from the other secondary outcome measures.
The BERLIN VT trial: Discussion
In summary, the BERLIN VT study found that preventive VT ablation before ICD implantation did not reduce the primary composite endpoint of allcause mortality and hospitalisation for symptomatic ventricular arrhythmia or worsening heart failure at 1 year follow-up. However, the patients in the preventive VT ablation group had fewer ventricular arrhythmias and ICD therapies compared to that of the deferred VT ablation group.
There were several important limitations of the trial. Firstly, like most VT ablation trials the patients and investigators were not blinded to the treatment allocation. Secondly, the small number of patients in both arms was secondary to the slow patient enrolment (similar to SMASH-VT and VTACH) as well as the high crossover rates (9.2% of patients in the preventive ablation group did not receive ablation while only 12% in the deferred group received ablation). In addition, not all the BERLIN VT investigators adhered to the protocol-defined timing of catheter ablation in the deferred ablation group for various reasons (only 2 patients underwent ablation after 3 ICD shocks as per protocol).
Therefore, the findings of BERLIN VT trial suggest that ischaemic VT ablation should be utilised only upon the recurrence of VT post-ICD implantation. This has the added benefit of avoiding exposure of patients to the risks of invasive catheter ablation.
Table 1 highlights the key findings of the VANISH and BERLIN VT trials.
| Table 1. Summary of the VANISH and BERLIN VT trials | ||
|---|---|---|
| Study | VANISH2 | BERLIN VT11 |
| Research question | Is catheter ablation of VT better than escalated AAD therapy in treating VT in ischaemic cardiomyopathy patients who already have an ICD? | When is the optimal time to perform VT ablation in ischaemic cardiomyopathy patients who are suitable for an ICD? 2 weeks before a planned ICD or wait until VT recurs? |
| Description | RCT comparing catheter ablation of VT vs escalated AAD therapy in ischaemic cardiomyopathy patients with ICD who had VT despite established AADs. | RCT comparing a ‘preventive’ VT ablation strategy at 2 weeks pre-ICD implantation vs ‘deferred’ VT ablation strategy after 3 ICD therapies in patients with stable ischaemic cardiomyopathy and VT. |
| Patient numbers | N=259. Ablation arm (N=132) vs escalated AAD arm (N=127) | N=159. Preventive ablation arm (N=76, 90.8% received ablation) vs Deferred ablation arm (N=83, 12% received ablation) |
| Primary outcome | VT ablation led to reduction in the composite primary endpoint of death, VT storm or appropriate ICD shock compared to escalation of AAD. Driven by a reduction in VT storm and appropriate ICD shock. | No difference in the composite primary endpoint of all-cause mortality and hospitalisation for heart failure or arrhythmia. |
| Other significant findings | If patients were already on amiodarone at baseline, the benefit in terms of the primary outcome was seen in the catheter ablation group. However, if patients were on a nonamiodarone AAD, then the benefit of catheter ablation was not observed. No difference in mortality between the two groups | Reduction in sustained VT and appropriate ICD therapy in the preventive ablation group. The large standard deviations and small patient numbers made it difficult to draw any further conclusions from the other secondary outcome measures |
| AAD antiarrhythmic drug, BERLIN VT Preventive Ablation of Ventricular Tachycardia in Patients with Myocardial Infarction, ICD implantable cardioverter defibrillator, MI myocardial infarction, RCT randomised controlled trial, VANISH Ventricular Tachycardia Ablation versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease, VF ventricular fibrillation, VT ventricular tachycardia. | ||
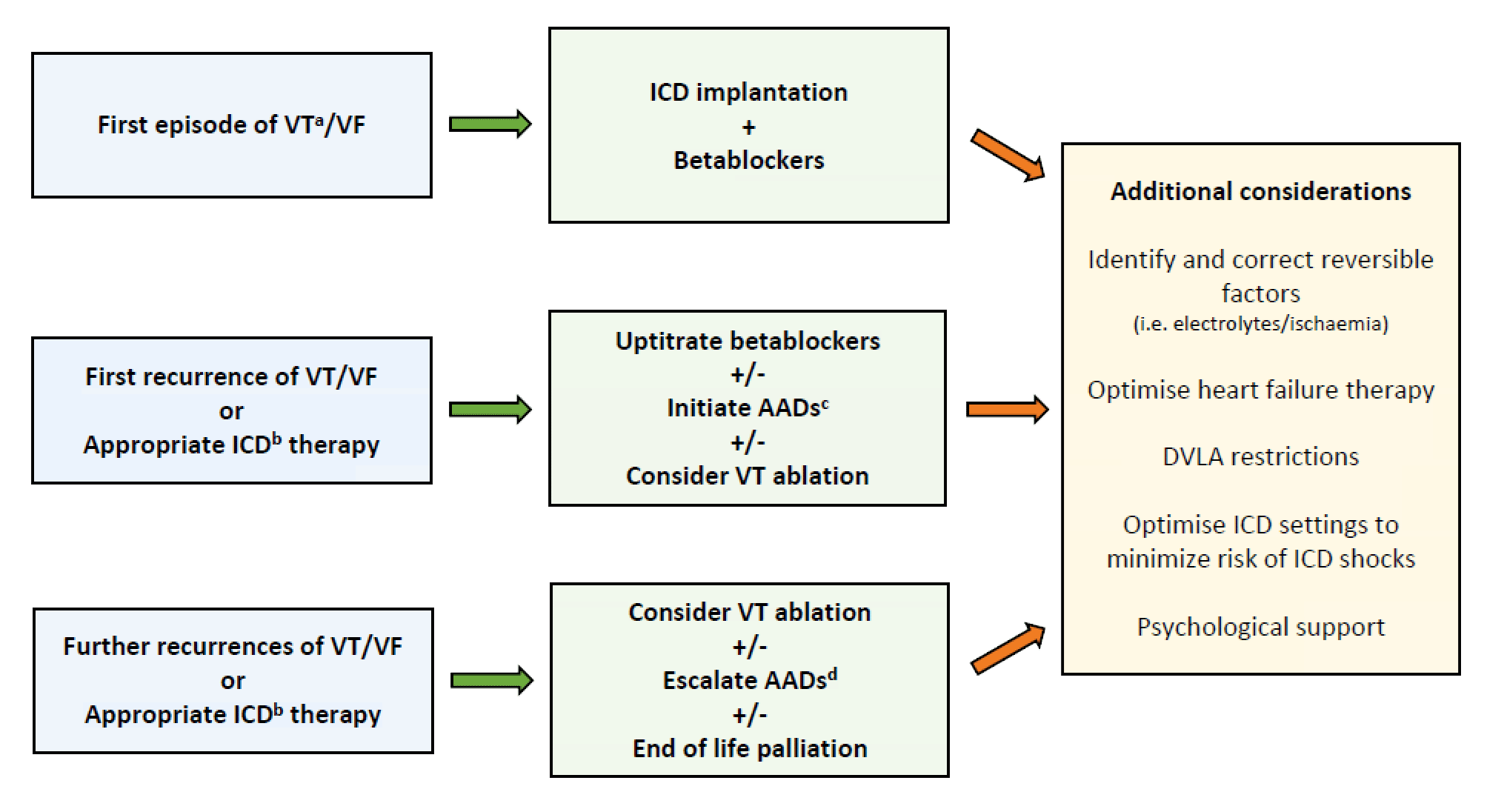
Figure 1. Suggested algorithm for the management of ischaemic ventricular tachycardia
a Sustained VT with haemodynamic compromise, b including anti-tachycardia pacing, ICD shocks or VT storm, c AADs defined as antiarrhythmic drugs excluding betablockers (e.g. in the UK typically Amiodarone is used first line, Sotalol or Mexiletine as second line), d Escalation protocol in the VANISH trial: if patient is already on Amiodarone <300mg OD then increase dose (loading dose 400mg BD for 2 weeks then 400mg OD for 1 week then 300mg OD as maintenance); if already on Amiodarone >300mg OD add Mexiletine (200mg three times daily); if patient on another AAD, then start Amiodarone (loading dose 400mg BD for 2 weeks, then 400mg OD for 4 weeks then 200mg OD as maintenance). AAD antiarrhythmic drugs, DVLA driver and vehicle licensing agency, ICD implantable cardioverter defibrillator, VT ventricular tachycardia, VF ventricular fibrillation.
Conclusion
The VANISH trial and the recently published BERLIN VT trial have helped shed light on the optimal timing of VT ablation as a secondary prevention treatment of VT in patients with ischaemic cardiomyopathy (see Figure 1). The positive findings from the VANISH trial have led to catheter ablation of VT recommended as the treatment of choice in ischaemic cardiomyopathy patients with an ICD who present with recurrent VT/ICD shocks despite AAD therapy (i.e. already taking Amiodarone or Sotalol).12 The BERLIN VT trial suggests that following index presentation, VT ablation provides no added benefit to ICD implantation but can be safely deferred until recurrence of VT/ICD therapies. Excitingly, more VT ablation trials are being conducted in this new decade and their findings will hopefully help refine patient selection and optimal timing of intervention as well as improve the efficacy and safety of ablation techniques.
Disclosures
None.
References
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. Eur Heart J 2000;21:2071–8.
- Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–21.
- Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DB, et al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families: A scientific statement from the American Heart Association. Circulation 2012;126:2146–72.
- Claro JC, Candia R, Rada G, Baraona F, Larrondo F, Letelier LM. Amiodarone versus other pharmacological interventions for prevention of sudden cardiac death. Cochrane Database of Systematic Reviews 2015;8(12):CD008093.
- Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, et al. Comparison of β-blockers, amiodarone plus β blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators - The OPTIC study: A randomized trial. J Am Med Assoc 2006;295:165–71.
- Pacifico A, Hohnloser SH, Williams JH, Tao B, Saksena S, Henry PD, et al. Prevention of implantable-defibrillator shocks by treatment with sotalol. N Engl J Med 1999;340:1855–62.
- Peachey H, Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, et al. EHRA/HRS/APHRS Expert Consensus on Ventricular Arrhythmias. Heart Rhythm 2014;11(10):e166–96.
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death-Executive Summary. J Am Coll Cardiol 2006;48(5):e247–346.
- Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–65.
- Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz E, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40.
- Willems S, Tilz RR, Steven D, Kääb S, Wegscheider K, Geller L, et al. Preventive or Deferred Ablation of Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT): A Multicenter Randomized Trial. Circulation 2020;141(13):1057-1067.
- Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;17(1):e2–154.
- Kuck KH, Tilz RR, Deneke T, Hoffmann BA, Ventura R, Hansen PS, et al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: Results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythmia Electrophysiol 2017;10(3):e004422.
- Hartzler GO. Electrode catheter ablation of refractory focal ventricular tachycardia. J Am Coll Cardiol 1983;2:1107–13.
Community Events Calendar


