The Impact of Poor Sleep on Atrial Fibrillation
| Take Home Messages |
|---|
|
Introduction
“A good laugh and a long sleep are the best cures in the doctor’s book” according to an old Irish proverb. Sleep has been described as the “elixir of life”1 – it is an essential component of optimal health and function. However, poor sleep habits and insufficient sleep are endemic in modern society, often precipitated by a combination of modern lifestyle, work patterns, technology and alcohol consumption2. Average sleep duration in the population has progressively shortened over recent decades3 and a UK study of over 2000 participants found that 37%reported difficulties with their sleep4.
Sleep is often neglected and misconceived as a dispensable activity. It is common to hear clichés such as “sleep is the enemy of success” or “you can sleep when you’re dead!”. In recent years however, there has been a renewed focus on the importance of sleep in mainstream media and public consciousness.
Chronic sleep deprivation causes adverse neurocognitive effects, metabolic disturbances and increased mortality3. There are significant cardiovascular consequences including increased risk of hypertension, myocardial infarction and arrhythmia3,5,6. In spite of this, the importance of sleep has not been fully embraced by the medical community3.
In this article, we will focus on the impact of sleep on atrial fibrillation (AF). Poor sleep is a plausible risk factor for AF, through direct influence on the pathogenesis of AF as well as affecting other established risk factors. 21% of patients in the I-STOP-AFib trial reported lack of sleep as a trigger for paroxysmal AF7. Experimental models suggest that sleep problems may contribute to the pathophysiology of AF through autonomic disturbance and induction of a pro-inflammatory state8.
Worldwide AF prevalence is growing and is projected to increase by more than 60% by 20509, due to an ageing population and growth in established risk factors such as obesity and diabetes. Known AF risk factors only account for a fraction of the population attributable risk10 and many patients have no identifiable risk factors11. International recommendations place huge importance on targeting modifiable lifestyle factors for primary and secondary prevention of AF12,13. Sleep may be an important risk factor for AF that could be more effectively targeted.
Sleep Disorders
Sleep disorders are highly prevalent and under-recognised. Conditions such as obstructive sleep apnoea (OSA), central sleep apnoea and restless legs syndrome (RLS) diminish sleep quality and have detrimental effects on the cardiovascular system14. OSA affects an estimated 1.5 million UK adults15, with rates likely to rise especially with increasing obesity rates16. Studies have shown OSA to be an independent risk factor for AF17,18 with the odds of AF increasing with increasing OSA severity19. Patients with OSA experience recurrent episodic airway obstruction resulting in negative intrathoracic pressure, hypoxaemia, pulmonary hypertension, disturbances of autonomic tone and sleep fragmentation16. These result in structural and electrical disturbances that promote atrial arrhythmogenesis (Figure 1).
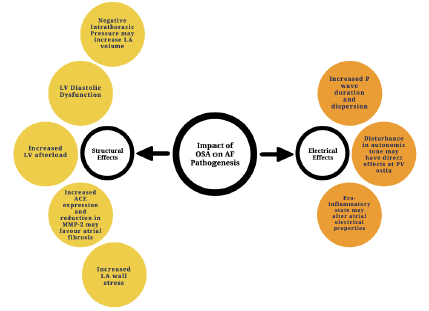
Figure 1. Potential factors influencing pathogenesis of AF in obstructive sleep apnoea16. ACE: angiotensin converting enzyme; LA: left atrium; LV: left ventricle; MMP-2: matrix metalloproteinase-2; PV: pulmonary vein.
Patients with AF may also have a high prevalence of undiagnosed OSA. In a study of 188 consecutive patients due to undergo catheter ablation for AF who did not have previous sleep apnoea diagnosis, patients were screened with a STOP-BANG questionnaire and a home sleep apnoea monitor20. 155 patients (82.4%) were positive for sleep apnoea on home testing and of these, 85 had moderate to severe disease warranting CPAP (continuous positive airway pressure) therapy. STOP-BANG questionnaire had a sensitivity of 81.2% and specificity of 42.4% for detection.
OSA is associated with increased AF recurrence following catheter ablation, and those with OSA may have significantly more non-pulmonary vein triggers compared to those without OSA16,21. In those in whom it is indicated, patients treated with CPAP therapy have greater post-ablation arrhythmia free survival compared to those not using it21. Though screening questionnaires are imperfect, these findings suggest that identification of co-existing OSA is important in the management of AF.
RLS is also associated with AF. In patients with AF and suspected RLS, frequent periodic leg movements during sleep have identified as an independent predictor of AF progression22. Treatment of RLS, for example with dopaminergic therapy, reduces the risk of AF incidence and progression22,23.
Sleep Characteristics Associated with AF
Sleep Disruption
There is evidence that sleep impairment outside of the context of sleep disorders may also promote AF. Sleep impairment may influence the risk of an acute episode of AF7 and lead to chronic changes that create an arrhythmogenic substrate for AF8.
A recent study examined the association between a healthy sleep pattern and risk of developing arrhythmia in over 400 000 participants from the UK Biobank5. Sleep pattern was given a score based on chronotype, sleep duration, insomnia, snoring and daytime sleepiness, from 0 (worst) to 5 (best). A healthy sleep pattern score was associated with a reduced risk of AF/atrial flutter. Those with a score of 5 (best) had a 29% reduced risk of developing AF compared to those scoring 0-1 (worst).
Another study investigating sleep characteristics associated with AF involved the analysis of patients in three large population-based studies for the association of sleep characteristics with the prevalence and incidence of AF24. The authors performed robust adjustment for features of OSA to separate this from other features of sleep. Investigators evaluated 4553 patients in the Heart eHealth study, which involved participants completing an online health questionnaire including information on sleep and AF status. Longer sleep onset latency (time to transition from wakefulness to sleep) and frequent night-time awakening was significantly associated with AF.
They subsequently looked at participants in the Cardiovascular Health Study (a prospective cohort study), finding that 28% of the 5703 patients had developed AF over a median follow-up of 11.6 years. Frequent night-time awakening predicted a 33% increased risk of AF incidence, independent of OSA diagnosis, and in a subset of 1127 patients in this study undergoing polysomnography, reduced duration of REM (rapid eye movement) sleep was also associated with increased risk of AF.
Investigators also evaluated data from the California HCUP study, which included a medical database of over 14 million patients with diagnosis codes from hospital admissions and attendances. A diagnosis of insomnia predicted a 36% increase in the risk of incident AF, which was a similar effect size to the risk of smoking (32%). OSA was associated with a 76% increased risk.
Together these findings suggest that healthy sleep pattern is protective against AF. Prolonged sleep onset latency, increased night-time awakening and reduced REM sleep may increase the risk of AF, independent of OSA.
Sleep Duration
Sleep duration may also be important. A retrospective study of 31709 patients who had undergone diagnostic polysomnography found that short sleep duration was independently associated with prevalent and incident AF25. Each 1-hour reduction in sleep duration correlated with a 17%increase in the odds of prevalent AF and 9%increase in incidence of AF. Further, each hour of reduction in REM sleep and N2 (stage two) sleep was associated with a 17% and 7% increased risk of incident AF, respectively. Other studies have suggested that both short sleep duration (<6 hours) and long sleep duration (>8 hours) may be associated with increased risk of AF26. Though exact recommendations for cut-offs are challenging to determine from these studies, under-sleeping or oversleeping seems to be detrimental.
Daylight Saving Time
Sleep impairment associated with clock changes for daylight saving may also increase the risk of AF. This was highlighted in a study on the impact of clocks going forward as a result of spring daylight saving time (DST)27. DST clock changes can result in sleep cycle derangement, disruption to circadian rhythm, sleep fragmentation, sleep latency and shortened sleep duration. There may be cumulative effects in the week following a clock change. Over an 8 year period in a US centre, overall mean daily admissions were significantly increased in the week (2.48 vs 2.09 admissions/day) following spring DST change, but not the autumn time change. When separated into gender, the finding was significant amongst women with a non-significant trend towards increased admissions in men. Whilst other unmeasured factors may have impacted on this trend, it suggests that the sleep impairment induced by DST could have a negative impact on AF.
Night-Shift Work
Night shift work disrupts the body’s circadian rhythm and disturbs metabolic and hormone pathways. Long-term night shift exposure may produce further cumulative effects. A recent cohort study divided 283 657 UK Biobank participants into four groups depending on the frequency or intensity of night-shifts28. Those undertaking usual/permanent night shifts had the highest risk of incident AF and there was a gradual increase in risk across the groups from day workers to usual/permanent night shifts. Those who undertook 3-8 nights/month of lifetime night shifts had a significantly higher risk of incident AF compared to those never working night shifts.
How Can We Improve Sleep?
There is a compelling case for the importance of sleep in atrial fibrillation and cardiovascular health. Increased awareness of the high prevalence of sleep problems and disorders can prompt further discussion during consultations. Conversations about sleep may stimulate patients to recognise its importance and to prioritise good sleep habits.
Clinical assessment may include determining the nature and chronicity of any sleep problems, the role of stress and lifestyle factors, and exploring any possible causes or symptoms suggestive of an underlying sleep disorder. Stress, alcohol and smoking are common factors influencing poor sleep. Nocturia is also significant cause of night-time awakening and is associated with worsened cardiovascular outcomes29.
There is a wealth of available information on good sleep hygiene (which refers to healthy sleep habits) that patients can be directed towards, including the websites of the British Heart Foundation30,31 and the Sleep Charity32. Public health initiatives and education also have a large role to play in improving sleep in the population. Ideal sleep is individual to each person but often includes a consistent sleep-wake schedule, sleeping for 8 hours in a comfortable environment with minimal disruption to sleep and avoiding the use of alcohol, nicotine and caffeine close to bed time. Sedative medications are not conducive to good quality sleep and are unsuitable for long-term use.
Digital technology can sometimes be a hindrance to healthy sleep habits. However, embracing digital initiatives is likely to be beneficial in tackling sleep problems. Website and app-based tools such as Sleepio™33 and Sleepstation34 are effective at improving sleep through education and cognitive behavioural therapy (CBT)35 and are available in some areas on the NHS. Smartwatches and monitoring devices may also be useful for some patients to monitor and improve their sleep. Additionally, dedicated pacemaker diagnostic algorithms may help in the identification of OSA in those with implanted cardiac devices.
Overall, a multi-faceted approach encompassing prompt identification of sleep problems and disorders, helping patients to improve sleep habits and a focus on ensuring adequate sleep duration, good sleep quality and minimising night-time awakening may be of benefit in prevention and treatment of AF.
Limitations
Many of the studies discussed are observational. Though adjustment was made for OSA in some studies, this is imperfect and OSA is often under-recognised. AF can be subclinical or asymptomatic and studies may have been prone to misrepresentation or under-reporting of AF, especially where self-reporting was involved.
Future Research
Research is needed to further clarify the exact role of sleep in the pathogenesis of AF. Additionally prospective, randomised studies into the impact of interventions such as improved sleep hygiene measures and CBT on improving AF are required to confirm their benefits in this population and support specific recommendations.
Conclusions
Poor sleep appears to be important in the pathogenesis of AF. OSA and other sleep disorders significantly impact on AF risk. Sleep abnormalities independent of OSA also appear to be an important contributor to AF, through sleep reduction, disruption and reduced sleep quality. Increased awareness may facilitate discussions and measures to improve sleep and consequently reduce the burden of AF. However, further research into the impact of specific sleep interventions on AF is needed to provide further insights into how sleep could be successfully targeted as a modifiable risk factor for AF in the future.
Disclosures
None.
References
- Sleep: The Elixir of Life (Website) https://insideangle.3m.com/his/blog-post/sleep-the-elixir-of-life/. Accessed online on 18/11/2021
- Chattu VK, Manzar MD, Kumary S et al. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare (Basel) 2019; 7(1):1
- Malhotra A and Loscalzo J. Sleep and Cardiovascular Disease: An Overview. Prog Cardiovasc Dis. 2009; 51(4):279-284
- Morphy H, Dunn KM, Lewis M et al. Epidemiology of Insomnia: a Longitudinal Study in a UK Population. Sleep 2007; 30(3):274-280
- Li X, Zhou T, Ma H et al. J Am Coll Cardiol 2021; 78:1197-1207
- Badran M, Yassin BA, Fox N et al. Epidemiology of Sleep Disturbances and Cardiovascular Consequences. Can J Cardiol 2015; 31(7):873-879
- Groh CA, Faulkner M, Getabecha S. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm 2019; 16(7): 996-1002
- Mehra R and Marcus GM. Chest 2019; 156(3): 421-423.
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke 2021; 16 (2):217-221.
- Schnabel RB. Can We Predict the Occurrence of Atrial Fibrillation. Clin Cardiol 2012;35 (1): 5-9
- Chugh SS, Blackshear JL, Shen WK et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001; 37(2):371-378
- Chung MK, Eckhardt LL, Chen LY et al. American Heart Association Electrocardiography and Arrhythmias Committee and Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Lifestyle and Cardiometabolic Health. Lifestyle and risk factor modification for the reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020; 141:e750-e772
- Visseren FLJ, Mach F, Smulders YM et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal 2021; 42: 3227-3337
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest 2017; 151(1):225-241.
- Obstructive Sleep Apnoea (OSA) – Toolkit for commissioning and planning local NHS services in the UK - 2015 (Website) https://www.blf.org.uk/sites/default/files/OSA_Tool kit_2015_BLF_0.pdf. Accessed online on 18/11/2021
- Maan A, Mansour M, Anter E et al. Obstructive Sleep Apnea and Atrial Fibrillation: Pathophysiology and Implications for Treatment. Crit Pathways in Cardiol 2015; 14:81-85.
- Gami AS, Hodge DO, Herges RM et al. Obstructive sleep apnea, obesity and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49:565-571
- Mehra R, Benjamin EJ, Shahar E et al. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910-916
- Kwon Y, Gharib SA, Biggs ML et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax 2015; 70:873-879
- Shapira-Daniels A, Mohanty S, Contreras-Valdes FM et al. Prevalence of Undiagnosed Sleep Apnea in Patients with Atrial Fibrillation and its Impact on Therapy. J Am Coll Cardiol EP 2020; 6:1499-1506
- Patel D, Mohanty P, DiBiase L et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnoea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445-451
- Mirza M, Shen WK, Sofi A et al. Frequent periodic leg movement during sleep is an unrecognized risk factor for progression of atrial fibrillation. PLoS One 2013; 8(10):e78359
- Gao X, Ba DM, Bagal K et al. Treating Restless Syndrome Was Associated With Low Risk of Cardiovascular Disease: A Cohort Study With 3.4 Years of Follow-Up. J Am Heart Assoc 2021;10:e018674
- Christensen MA, Dixit S, Dewland TA et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm 2018; 15:1289-1295
- Genuardi MV, Ogilvie RP, Saand AR et al. Association of Short Sleep Duration and Atrial Fibrillation. Chest 2019; 156(3):544-552
- Morovatdar N, Ebrahimi N, Rezaee R et al. Sleep Duration and Risk of Atrial Fibrillation: a Systematic Review. J Atr Fibrillation 2019; 11(6):2132-2137
- Chudow JJ, Dreyfus I, Zaremski L et al. Changes in atrial fibrillation admissions following daylight saving time transmissions. Sleep Medicine 2020; 69:155-158
- Wang N, Sun Y, Zhang H et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. European Heart Journal 2021; 42:4180-4188.
- Parthasarathy S, Fitzgerald MP, Goodwin JL et al. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One 2012; 7:1-8.
- Sleeping Tips – British Heart Foundation (Website) Accessed on 13/11/2021. https://www.bhf.org.uk/informationsupport/heart-matters-magazine/wellbeing/sleeping-tips. Accessed on 13/11/2021.
- How does sleep affect your heart? – British Heart Foundation (Website) https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2019/november/sleep-and-heart-and-circulatory-diseases. Accessed on 13/11/2021.
- The Sleep Charity (Website) https://thesleepcharity.org.uk. Accessed on 13/11/2021.
- Sleepio (Website) https://www.sleepio.com. Accessed on 18/11/2021.
- Sleepstation (Website) https://www.sleepstation.org.uk/nhs_options/. Accessed on 18/11/2021.
- Espie CA, Kyle SD, Williams C et al. A randomized, placebo-controlled trial of online cognitive behavioural therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep 2012; 35(6):769-781
