The future of lithotripsy – not just for the kidneys
| Take Home Messages |
|---|
|
Introduction
In 1929 Werner Forssman a German physician conducted the first heart catheterisation by passing a urinary catheter tube into his own cubital vein and into his right heart under X ray guidance.(1) Thus, the concept of heart catheterisation began. 90 years later, urology has once again inspired the future of cardiology with the novel concept of intravascular lithotripsy for severe coronary calcification. For the past 25 years lithotripsy waves have been used to dissolve kidney stones, now this same technology is being used to dissolve calcium in the coronary arteries in order to achieve optimal results during coronary intervention.
This editorial aims to highlight the current evidence behind this brand new technology in the field of coronary intervention.
Background
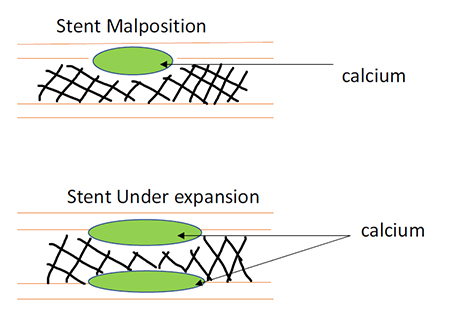
As the population ages, the number of coronary lesions with significant vascular calcium is increasing. Severe calcification is most commonly defined as radiopacities seen without cardiac motion before contrast injection, usually affecting both sides of the arterial lumen.(2)
There are a number of challenges to coronary intervention as a result of this and these include: the requirement for higher pressure to dilate effectively, increased number of dissections, decreased stent expansion and increased complications resulting in poorer clinical outcomes.(3) The current available methods to tackle calcified lesions include the use of various compliant, non-compliant and cutting balloons and rotational atherectomy, however results with these are not always optimal and without risk.
Risk factors for increased levels of calcification are listed in the table below:(4)
| RISK FACTOR | INTIMAL CALCIFICATION | MEDIAL CALCIFICATION |
|---|---|---|
| Advanced age | Yes | Yes |
| Diabetes Mellitus | Yes | Yes |
| Dyslipidaemia | Yes | No |
| Hypertension | Yes | No |
| Male | Yes | No |
| Cigarette smoking | Yes | No |
| Renal aetiology | ||
| Decreased eGFR | No | Yes |
| Hypercalcaemia | No | Yes |
| Hyperphosphataemia | Yes | Yes |
| PTH abnormalities | No | No |
| Duration of dialysis | No | Yes |
Table 1: Risk factors for developing coronary calcification
Current devices used to better understand the extent of calcification prior to percutaneous coronary intervention include intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
Intravascular ultrasound is substantially more accurate with a sensitivity of 90% to 100% and specificity of 99% to 100%.(5) The extent of calcification can be graded, and the length measured, the only drawback is that thickness and volume of the calcified segment cannot be fully assessed due to the inability of ultrasound to penetrate the calcium.
Optical coherence tomography provides higher-resolution imaging than grayscale IVUS and detects calcium as low-intensity, low-attenuation areas with sharp borders.(6) OCT can also be utilised in many cases to assess calcium thickness and measure calcium volume.(7)
Utilising the above technologies, sometimes also in combination with each other, lesions can be better characterised. However, this does not solve the problems that arise during intervention in these circumstances. Cutting and scoring balloons do not remove calcium, they simply improve vessel compliance by creating incisions, enabling greater lesion expansion and reducing recoil while preventing uncontrolled dissections.(8) These carry a higher risk of vessel perforation and myocardial infarction.(9)
Rotational atherectomy on the other hand actually does ablate coronary calcium using a device with a diamond-coated elliptical burr. This device abrades hard tissue into smaller particles and deflects off softer tissue.(10) However the disadvantages are that patients are at higher risk of thrombus formation, slow or no reflow, peri-procedural myocardial infarctions and microembolisation.(11)
So, what is the solution for these high-risk lesions in the increasingly aged population we are frequently encountering in the cath lab, and in whom coronary bypass grafting may also not be an option?
SHOCKWAVE LITHOTRIPSY!
The Shockwave Coronary Lithoplasty System is a balloon catheter system designed to deliver localised, lithotripsy-enhanced, balloon dilatation of calcified coronary arteries. Energising the lithotripsy electrodes generates a pulsatile mechanical energy within the target treatment site and this disrupts calcium within the lesion and allows subsequent dilatation of a coronary artery stenosis using low balloon pressure. The system consists of a rapid exchange balloon catheter with integrated, internal lithotripsy electrodes and a Shockwave generator.(12)
The Initial Study
The DISRUPT-CAD(13) study was a multicentre, prospective, single-arm, open label study of percutaneous lithoplasty prior to stent implantation in heavily calcified coronary lesions. The study enrolled 60 patients across the world with stable angina, unstable angina or silent ischaemia. Patients had moderate to severely calcified de novo coronary lesions, RVD 2.5 – 4.0mm, with stenosis >50% and a lesion length less than or equal to 32mm. The objective was to assess the safety and performance of the Shockwave Medical Coronary Rx Lithoplasty® System. The primary safety endpoint was MACE within 30 days defined as: cardiac death, myocardial infarction or target vessel revascularisation. The primary performance endpoint clinical success was defined as residual stenosis (<50%) after stenting with no evidence of in-hospital MACE.
The results demonstrated that there were no periprocedural angiographic complications (including dissections, perforations and slow flow). There was 95% clinical success with 57/60 lesions having residual stenosis of <50% and 98.3% device success and delivery at target lesion.
Real World Data
The most recent publication in March 2019 in the Journal of Invasive Cardiology looks at real world evidence from the use of the lithotripsy balloon. 14 From October 2018 – January 2019 a total of 26 patients undergoing PCI were treated with S-IVL prior to stent deployment (69% male; age, 72 ± 8 years). Indications for PCI were acute coronary syndromes (ACS) in 14 patients (54%), stable angina in 11 patients (42%), and PCI before transcatheter aortic valve implantation in 1 patient (4%). Seventy-one percent of the ACS cases undergoing PCI with S-IVL were to the perceived ACS culprit lesion during the index procedure, while 29% were staged PCIs to severe non-culprit lesions. Upfront S-IVL usage occurred in 58% of cases; the rest were bail-out procedures due to suboptimal initial balloon predilation. S-IVL was used most commonly in the left anterior descending coronary artery (50%), with 1.3 ± 0.5 stents implanted/target vessel. Angiographic success (<20% residual stenosis) occurred in all cases, with no procedural complications. From this study and the evidence so far, shockwave lithotripsy appears to be a useful modality in coronary calcium modification to optimise stent expansion.
Conclusion
As the population ages and patients become more medically complex, increased coronary calcification will persist and such lesions will present challenges to the interventional cardiologist. This new technology of shockwave lithotripsy appears set to revolutionise the approach and management of severely calcified coronary lesions. More work needs to be done in a larger patient population and in comparison to current available methods to deal with calcified lesions, however the future looks promising. One wonders what the next urological inspiration for cardiologists might be?
References
- https://en.wikipedia.org/wiki/Werner_Forssmann#cite_ref-wiley_3-0 - Last accessed 15/03/2019
- J. Popma, T. Bashore. Qualitative and quantitative angiography Textbook of Interventional Cardiology, WB Saunders, Philadelphia, PA (1994), pp. 1052-1068
- Mintz et al. J Am Coll Cardiol 2014;64(2):207-222
- W.G. Goodman, G. London, K. Amann, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis, 43 (2004), pp. 572-579
- G.J. Friedrich, N.Y. Moes, V.A. Muhlberger, et al. Detection of intralesional calcium by intracoronary ultrasound depends on the histologic pattern Am Heart J, 128 (1994), pp. 435-441
- H. Yabushita, B.E. Bouma, S.L. Houser, et al. Characterization of human atherosclerosis by optical coherence tomography Circulation, 106 (2002), pp. 1640-1645
- T. Kume, H. Okura, T. Kawamoto, et al. Assessment of the coronary calcification by optical coherence tomography EuroIntervention, 6 (2011), pp. 768-772
- P. Barath, M.C. Fishbein, S. Vari, J.S. Forrester Cutting balloon: a novel approach to percutaneous angioplasty. Am J Cardiol, 68 (1991), pp. 1249-1252
- J.A. Bittl, D.P. Chew, E.J. Topol, D.F. Kong, R.M. Califf. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J Am Coll Cardiol, 43 (2004), pp. 936-942
- M. Zimarino, T. Corcos, E. Bramucci, C.Tamburino. Rotational atherectomy: a “survivor” in the drug-eluting stent era Cardiovasc Revasc Med, 13 (2012), pp. 185-192
- T. Kume, H. Okura, T. Kawamoto, et al. Assessment of the histological characteristics of coronary arterial plaque with severe calcification Circ J, 71 (2007), pp. 643-647
- https://clinicaltrials.gov/ct2/show/NCT02650128 - Last accessed 17/3/2019.
- https://www.acc.org/~/media/Clinical/PDF-Files/ApprovedPDFs/2016/10/25/07/TCT16_Oct29/12pmET%20DISRUPT%20CAD.pdf - Last accessed 17/3/2019.
- B Wong, S El-Jack, R Newcombe et al. Shockwave Intravascular Lithotripsy for Calcified Coronary Lesions: First Real-World Experience. J Invasive Cardiol. 2019 Mar;31(3):46-48. Epub 2019 Feb 15.
Community Events Calendar


