Smartwatches: The New Way to Diagnose Arrhythmias?
| Take Home Messages |
|---|
|
Introduction
Consumer spending on smartwatches is increasing year on year. In 2020, the smartwatch market was worth $21.8 billion with further growth projected to $31.3 billion in 20221. Common technological features on smartwatches now include collection of biometric data such as heart rate. In 2018, the Apple Watch Series 4® was the first to take this a step further, being able to produce a single lead electrocardiogram (ECG). Since then, other companies including Samsung®, FitBit®, Withings® and Amazfit® have produced ECG capable smartwatches (hereafter referred to as ‘ECG watches’).
The technology
At time of writing, all smartwatches obtain a heart rate using photoplethysmography. Photoplethysmography is most seen medically in an oxygen saturation probe allowing for reading of both oxygen saturations and heart rate. Varying numbers of LEDs and photodiodes placed on the back of a smartwatch emit and measure the amount of reflected light which will vary with a peripheral pulse. From this a ventricular rate is calculable2.
Although it may be subtly different between watch brands, producing a single lead ECG essentially requires the same process. Part of the ECG watch acts as an electrode (e.g. the ‘Digital Crown®’ on the Apple Watch) which is then touched by the user’s opposite hand. This forms two unipolar readings, one from the heart via the ipsilateral arm to the watch, and one from the heart via the contralateral arm to the electrode. The difference between these unipolar readings generates a bipolar lead which is synonymous with lead 1 on a 12 lead ECG3. After touching this electrode for 30-60 seconds at rest, the ECG is available for review alongside a report on a smartphone app.
ECG watches by Apple®, Samsung®, and Fitbit® all have Conformité Européene (CE) marks for Europe and Food and Drug Administration (FDA) approval in the USA. Their approval allows for the running of proprietary algorithms to report the ECG rhythm stating sinus rhythm (SR), atrial fibrillation (AF) or inconclusive. Currently, Withings® have a CE Mark and are expecting FDA approval, whilst Amazfit as the most recently released device has neither but is undergoing the approval process.
All ECG watches have a disclaimer stating they are not designed to diagnose myocardial infarction nor other medical conditions. They also state not to change medications based upon results and to seek medical attention if the user is unwell.
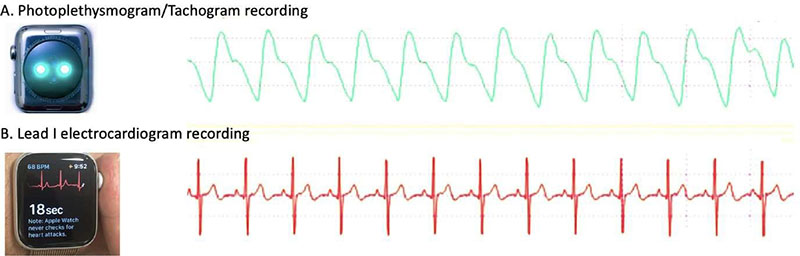
Figure 1. Demonstration of a tachogram and Lead 1 ECG recording from Apple Watch®. (Figure from Isakadze et al4)
ECG watches can accurately diagnose AF
AF is the most common cardiac arrhythmia affecting just under 1.5 million people in the United Kingdom5. AF is associated with increased risks of death6, stroke7, heart failure8, dementia9, decreased quality of life10,11 and places large financial and societal cost on the NHS12. Due to this, accurate diagnosis and treatment of subclinical AF with anticoagulation could improve outcomes. However, diagnosing AF can be challenging, with 59.5% of patients presenting with atypical symptoms or none at all13. Furthermore, AF is a progressive disease beginning in a paroxysmal form14, meaning that even if symptoms are present, detection on intermittent monitoring devices requires a degree of serendipity. Lastly and perhaps most concerningly, patients with asymptomatic or atypical AF presentations have higher risks of stroke compared to typical presentations even when accounting for co- morbidities or anticoagulation13,15.
Consequently, a simple, non-invasive method to identify subclinical AF could provide significant public health benefits. Potentially, this method could use ECG watches as a solution.
Currently, ECG watches still require patients to manually create an ECG. However, Apple® ECG watches have an algorithm that identifies an irregular pulse automatically. For 1 minute every 2 hours, the watch will create a tachogram (a graphical recording of blood flow). If background noise is minimal, and the peak to peak intervals on the tachogram are irregular, a Poincare plot is generated, and dispersion analysed. Through this the irregularity of the pulse is assessed in greater detail. If the pulse is deemed to be irregular, the watch will then sample up to every 15 minutes for 48 hours. If 5 of 6 consecutive tachograms are found to be irregular, the watch will notify the user, an ECG can be taken, and AF potentially identified16. From this design the algorithm will not detect short episodes of AF, and acts to minimise false positives.
To validate this technology and algorithm, the ECG 1.0 Clinical Validation Study was performed3. There were two aspects to this study. Firstly, visually comparing the morphology of the ECG generated by the watch to that of lead 1 of a 12 lead ECG, through simultaneous recordings. Secondly, to establish the sensitivity and specificity of the Apple Watch® algorithm to identify SR or AF by comparison to a 12 lead ECG assessed by 3 cardiologists.
301 patients with AF and 287 with SR met eligibility criteria. For morphological comparison the ‘pass rate’ was high for both AF (60/61, 98.4%) and SR (65/65, 100%), validating the watch generated ECG as an accurate reflection of lead 1. The sensitivity and specificity for rhythm analysis was also impressive. For AF, the algorithm correctly identified the rhythm in 239/263 (90.9%) of cases, with the remainder as ‘unreadable.’ For SR, the rhythm was identified correctly in 232/244 (95.1%) of cases, with 10 unreadable and 2 classified as ‘other’. Consequently, for classifiable rhythm strips achieved by the algorithm, the sensitivity and specificity for rhythm identification was 98.3% and 99.6% respectively.
The ECG 2.0 Validation Study3 expanded on the above, again analysing the sensitivity and specificity of an updated algorithm, but this time expanding the heart rate range to 50 - 150bpm and possible categories to SR, AF, SR with high heart rate, AF with high heart rate, inconclusive and poor recording. Once again, the results were impressive with the overall classifiable ECG recordings showing a 98.5% sensitivity and 99.3% specificity. It was noted that with higher heart rates (100 – 150 bpm), the algorithm was less successful with sensitivities of 90.7% and 83% for SR and AF respectively.
One must note that although these results are impressive, they were performed in a controlled setting with technicians ensuring the ECG watch was correctly placed and ECGs collected with the correct technique. Expanding to a population of watch users is likely to yield a greater number of unclassifiable results. Regardless, these two ECG Validation Studies did demonstrate the rhythm identification capabilities of the Apple Watch®.
Results from these studies ultimately led to FDA approval and a CE mark for the Apple Watch® algorithm. Results from studies from other companies were unavailable at time of writing, but did lead to the relevant approvals.
In the real world, the Cleveland Clinic investigated the sensitivity and specificity of the Apple Watch Series 4® (i.e. ECG capable) in diagnosing AF in 50 patients post cardiac surgery17. This group was selected due to their high incidence of AF and compared to telemetry. The watch was placed on each patient 3 times a day and two recordings taken each time. The authors report a 41% sensitivity (34/90) and 100% specificity for AF detection by the watch. This is a significant decrease compared to the controlled environment studies above. However, this acute scenario is not one for which the watch was intended, and the study does not report the ventricular rates experienced by patients while in AF. The sensitivity of the watch is known to decrease with increasing ventricular rate (often seen post cardiac surgery) which could explain the results here. Interestingly, the single lead ECG PDF file produced and stored by the watch demonstrated AF accurately in 93% of circumstances. The remaining 7% failed to generate the file. The results suggest that although the watch may not be able to diagnose AF directly with high accuracy in this scenario, the ECG PDF file it produces stores potentially very useful clinical information.
Identifying AF in the Real World
Moving outside of a controlled environment, studies using ECG watches in the real world are small in number reflecting the novelty of the technology.
The largest study to assess the ability of a smartwatch to identify an irregular pulse is the Apple Heart Study16. The Apple Heart Study was a first of its kind study being siteless and using a smartphone app to advertise to and recruit participants. With this, a huge 419,297 participants without known AF were recruited over 8 months. The study sought to estimate the proportion of participants who, having received a notification of an irregular pulse, genuinely had AF. Assessment for AF was via a 7 day ECG patch sent to participants by the investigators. As the study used earlier versions of the Apple Watch, real time ECG capabilities were not available, and instead was reliant upon data from tachograms.
2161 (0.52%) participants received an irregular pulse notification. 658 had a 7-day ECG patch sent to them to try and confirm AF. 450 patches were returned, and AF was confirmed in 34%. Interestingly, of those patients who received an irregular pulse notification whilst the patch was worn, the positive predictive value for AF of the notification was 84%16. 89% of participants with AF had an episode of greater than 1 hour. The authors concluded that the probability of having an irregular pulse is low, but of those who do a significant number were diagnosed with AF.
The study has several limitations. Although the number of participants was high the number of irregular pulse notifications was low and only 450 of 658 ECG patches were returned. Consequently, the authors were unable to reach the targeted statistical precision desired. 66% of returned ECG patches did not show AF. However, the median time between notification and ECG patch recording was 13 days. Consequently, the reason for pulse notification was not captured in real time and could represent underreporting of paroxysmal AF which was missed. Alternatively, whether the notifications represented false positives or other clinically relevant arrhythmias could not be confirmed.
The study was not intended to be a population-wide screening study for AF. Indeed, the demographic studied were smartwatch (and iPhone®) owners, both of which had been bought voluntarily. They were predominantly younger (52% aged 22-39), male (58%), and white (68%). As evidenced by only 12% having a CHA2DS2-VASc score of 2 or above, the study did not target high risk groups for AF, nor did it reflect societal demographics as a whole. Furthermore, the study did not identify the true sensitivity of the ability of the Apple Watch to identify AF. To truly undertake this, ECG patches would have had to be sent to all participants, something for which the trial was not designed.
However, the innovative and pragmatic nature of the Apple Heart Study lays a potential blueprint for studies in the future. It showed that its algorithm for detecting an irregular pulse had the potential to identify subclinical AF and that huge studies could be conducted using internet connected devices.
The Heartline study18 is currently recruiting and seeks to answer if use of an ECG watch is able to improve AF diagnosis and clinical outcomes. 150,000 participants without known AF, aged 65 or above will be randomised to using an Apple ECG Watch® or standard of care (no watch) to look for differences in time to diagnosis of AF and major adverse cardiovascular events. A second arm of the study will recruit 10,000 participants with AF and randomise to use of an Apple ECG Watch with an ‘adherence module’ or no watch to look for improvements in compliance to anticoagulation. The Heartline study is currently estimated to complete in June 2024 and will be the first to provide large scale data on specific use of ECG watches in high risk population in diagnosis and treatment outcome of AF.
AF Population Screening
Although early in their use, studies have demonstrated ECG watches to have the ability to recognise an irregular pulse and determine AF in both controlled and real-world settings. The potential to upscale these findings to develop an AF screening programme to mitigate the mortality and morbidity associated with AF is attractive. However, there is currently incomplete evidence that an AF screening programme would bring about significant public health benefits.
Pre-publication results of the STROKESTOP study were recently presented at the 2021 European Heart
Rhythm Association (EHRA) virtual conference19. The study invited 13,979 participants aged 75-76 in two regions of Sweden to participate in an AF screening programme and compared against 13,996 who were not. Of those invited for screening, 7,173 (53%) took part. By age alone, all participants would have had a CHA2DS2-VASc of at least 2 and be candidates for anticoagulation. Twice daily single lead ECGs via a handheld device were obtained in the screening group over 14 days. 3.0% of those screened were found to have previously unknown AF. These participants were counselled and offered anticoagulation, which was taken up by 93%20.
The primary endpoint was a composite of ischaemic stroke, systemic thromboembolism, haemorrhagic stroke, and hospitalisation for bleeding. After over
7 years of follow up, the primary endpoint was found to be significantly lower in the screening group (HR 0.96, p = 0.045), driven mainly by a decrease in ischaemic stroke (HR 0.76, 95% confidence interval 0.68 – 0.87). Interestingly the event curves only began to separate after 4 years, suggesting long term compliance is required to see significant results.
As incident rates of the primary endpoint were low, the difference between screened and non-screened groups was only just significant, a cost effectiveness analysis would be required to see if population screening in this manner would be economically viable.
ECG Watches could improve AF detection
The STROKESTOP study suggests a significant clinical benefit in AF screening in an elderly population. However, the intensive screening required compliance of the participants to perform bidaily ECGs for 14 days. ECG watches with algorithms to passively detect an irregular pulse followed by a potential real time ECG could offset this need for compliance and bring costs down. However, ECG watches themselves would require compliance in being worn with less than 50% of consumers using theirs after 1.5 years21.
Furthermore, a significant cost is involved in owning a smartwatch leading to social disparities in availability of screening. One could also hypothesise that owners, particularly those with additional health-based apps, are more likely to be health conscious individuals, less likely to experience adverse events. The challenge is to engage with the population who are not, and indeed those who were invited for, but declined screening in STROKESTOP had more co-morbidities, higher CHA2DS2-VASc scores and had higher rates of ischaemic stroke than even those who were not invited for screening19.
Further possibilities
The diagnostic algorithms used by ECG watches are currently intended to detect previously undiagnosed AF. They are not intended for use by patients with a known diagnosis of AF. However, certain patient subgroups could find their AF detection features particularly useful. Patients who have undergone rhythm control such as DC cardioversion or ablation to treat AF would be able to monitor their rhythm closely following a procedure when recurrence is known to be higher. Following self-diagnosis, PDFs of the rhythm could be sent to their heart rhythm team for review and management plans such as commencing anti-arrhythmics could be initiated swiftly. Likewise, patients with paroxysmal AF currently using a pill in the pocket strategy could gain reassurance from their ECG watch in confirming an episode of AF and commencing therapy. Both these strategies also help enhance patient’s knowledge of their disease, empower self- management and could prevent unnecessary hospitalisation.
Although not licensed to recognise other arrhythmias, myocardial infarction or other ECG abnormalities, the 1 lead ECG generated by ECG watches has repeatedly been shown to be a reliable representation of lead13,17. Theoretically, the PDF files with these ECGs could be used for diagnosis of other cardiac conditions by a qualified medical professional accepting the limitations of a single lead. Indeed, atrial flutter22, ventricular tachycardia23, supraventricular tachycardia24, and Mobitz 2 heart block25 have all been diagnosed via ECG watches to the benefit of their users. Furthermore, a 3 lead ECG capable of diagnosing ST elevation myocardial infarction has been shown to be possible, even if it requires moving the watch to the ankles and is perhaps unnecessary26.
Lastly, ECG watches provide a potential method for diagnosing infrequent arrhythmias without the need for an implantable loop recorder. Although ECG watches require a patient to take their own ECG and thus introduce a delay, the possibility of avoiding an invasive procedure will appeal to some patients. This benefit of course would not extend to those being investigated for cardiac syncope.
Data Ownership
With the advent of smartwatch health apps, the amount of personal health data collected and transmitted into the cloud is ever increasing. The raw data generated by ECG watches are anonymised and encrypted on the device, but owned by the respective companies. There is the option to upload the data to online servers. There is also the ability to deactivate rhythm monitoring entirely. This brings into question the ethics and legal responsibilites of who owns and processes this data and the potential for confidentiality breaches.
Conclusions
Smartwatches can accurately assess for an irregular pulse.
ECG watches can accurately generate a single lead ECG and diagnose AF.
ECG watches can also store other arrhythmias, the interpretation for which they are unlicensed.
There is the potential for ECG watch owners to be screened for AF but this magnifies pre-existing sociodemographic disparities and requires habitual long term use.
The evidence for screening the whole population for AF regardless of method is incomplete and questions remain about its economic viability.
Disclosures
None.
References
- Gartner Inc. Gartner Forecasts Global Spending on Wearable Devices to Total $81.5 Billion in 2021. (Date accessed 30/4/2021) https://www.gartner.com/en/newsroom/press-releases/2021-01-11-gartner-forecasts-global-spending-on-wearable-devices-to-total-81-5-billion-in-2021#:~:text=Worldwide%20end%2Duser%20spending%20on,latest%20forecast%20from%20Gartner%2C%20Inc.2021
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1-39.
- Apple Inc. Using Apple Watch for Arrhythmia Detection: Apple Inc. Accessed 30/4/2021.; 2020 [Available from: https://www.apple.com/ca/healthcare/docs/site/Apple_Watch_ Arrhythmia_Detection.pdf.]
- Isakadze N, Martin SS. How useful is the smartwatch ECG? Trends in Cardiovascular Medicine. 2020;30(7):442-8.
- Public Health England. Atrial Fibrillation Prevelance Estimates 2019 [cited 2021. Available from: https://www.gov.uk/government/publications/atrial-fibrillation- prevalence-estimates-for-local-populations.]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-52.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N-9N.
- Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695-703.
- Saglietto A, Matta M, Gaita F, Jacobs V, Bunch TJ, Anselmino M. Stroke-independent contribution of atrial fibrillation to dementia: a meta-analysis. Open Heart. 2019;6(1):e000984.
- Peinado R, Arribas F, Ormaetxe JM, Badia X. Variation in quality of life with type of atrial fibrillation. Rev Esp Cardiol. 2010;63(12):1402-9.
- Steg PG, Alam S, Chiang CE, Gamra H, Goethals M, Inoue H, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98(3):195-201.
- Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90(3):286-92.
- Siontis KC, Gersh BJ, Killian JM, Noseworthy PA, McCabe P, Weston SA, et al. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: Characteristics and prognostic implications. Heart Rhythm. 2016;13(7):1418-24.
- Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14(6):801-7.
- Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol. 2013;168(5):4744-9.
- Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. New England Journal of Medicine. 2019;381(20):1909-17.
- Seshadri DR, Bittel B, Browsky D, Houghtaling P, Drummond CK, Desai MY, et al. Accuracy of Apple Watch for Detection of Atrial Fibrillation. Circulation. 2020;141(8):702-3.
- ClinicalTrials.gov. A Study to Investigate if Early Atrial Fibrillation (AF) Diagnosis Reduces Risk of Events Like Stroke in the Real-World, Accessed 06/05/2021 2021 [Available from: https://clinicaltrials.gov/ct2/show/NCT04276441.]
- Svennberg E, editor Benefits of systematic screening for atrial fibrillation – the STROKESTOP study. . Presented at: EHRA 2021; 2021 23/4/2021; Virtual.
- Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131(25):2176-84.
- Eapen ZJ, Turakhia MP, McConnell MV, Graham G, Dunn P, Tiner C, et al. Defining a Mobile Health Roadmap for Cardiovascular Health and Disease. J Am Heart Assoc. 2016;5(7).
- Ahmed AS, Golden KM, Foreman JR, Padanilam BJ. Using a smartwatch to identify the morphology of atrial flutter. HeartRhythm Case Rep. 2020;6(10):808-9.
- Burke J, Haigney MCP, Borne R, Krantz MJ. Smartwatch detection of ventricular tachycardia: Case series. HeartRhythm Case Rep. 2020;6(10):800-4.
- Hwang J, Kim J, Choi KJ, Cho MS, Nam GB, Kim YH. Assessing Accuracy of Wrist-Worn Wearable Devices in Measurement of Paroxysmal Supraventricular Tachycardia Heart Rate. Korean Circ J. 2019;49(5):437-45.
- Walsh KA, Lin D. A Smartwatch Heart Rate Monitor Prompts an Unusual Diagnosis. JACC: Case Reports. 2020;2(3):431-3.
- Avila CO. Novel Use of Apple Watch 4 to Obtain 3-Lead Electrocardiogram and Detect Cardiac Ischemia. Perm J. 2019;23.
Community Events Calendar


