Risk screening in cardiovascular disease: the role of CT coronary angiography in asymptomatic patients, why wait?
| Take Home Messages |
|---|
|
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality in the UK, and importantly coronary artery disease (CAD) is the most common cause of premature and avoidable death in the UK. The burden of its management and treatment requires substantial health-care resources and places a burden on patients, families and the healthcare
system. The NHS Long Term Plan, published in 2019, recognised this burden and stated that it was “the single biggest area where the NHS can save lives over the next 10 years”. It recognised that the “early detection and treatment of CVD can help patients live longer, healthier lives. Too many people are still living with undetected, high-risk conditions”, and within their report they set out a series of goals, including increasing work on the prevention of heart attacks.
The role of screening programmes in the prevention and detection of early disease across healthcare
settings and specialties continues to expand. Optimising screening strategies enables potential intervention earlier in disease pathways to improve patient outcomes. Criteria outlined in the seminal Wilson and Jungner WHO report “Principles and practice of screening for disease”3, issued in 1968, have long been considered the gold standard in assessing the appropriateness of a screening tool. Their criteria, alongside a suggested modified version issued in 20084, are listed in Box 1. CVD clearly meets many of both the original and emerging screening criteria. However, in this editorial will discuss whether the current screening strategy would benefit from the addition of computed tomography coronary angiography (CTCA).
| Box 1. | |
|---|---|
| Wilson and Jungner Screening Criteria | Emerging screening criteria proposed |
| 1. The condition sought should be an important health problem | 1. The screening programme should respond to a recognised need |
| 2. There should be an accepted treatment for patients with recognised disease | 2. The objectives of screening should be defined at the outset |
| 3. Facilities for diagnosis and treatment should be available | 3. There should be a defined target population |
| 4. There should be a recognisable latent or early symptomatic stage | 4. There should be scientific evidence of screening programme effectiveness |
| 5. There should be a suitable test or examination | 5. The programme should integrate education, testing, clinical services and programme management |
| 6. The test should be acceptable to the population | 6. There should be quality assurance, with mechanisms to minimize potential risks of screening |
| 7. The natural history of the condition, including development from latent to declared disease, should be adequately understood | 7. The programme should ensure informed choice, confidentiality and respect for autonomy |
| 8. There should be an agreed policy on whom to treat as patients | 8. The programme should promote equity and access to screening for the entire target population |
| 9. The cost of case finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole | 9. Programme evaluation should be planned from the outset |
| 10. Case finding should be a continuing process and not a “once and for all” project | 10. The overall benefits of screening should outweigh the harm |
CVD risk assessment: current clinical guidance
CVD prevention is delivered both at the general population level (promoting healthy lifestyle behaviour), and at the individual level by tackling unhealthy lifestyles and by targeted pharmacotherapy for causal CV risk factors. Modification of reversible risk factors (rather than interventional procedures) has been largely responsible for the decline in age-adjusted cardiovascular mortality5.
Both National Institute for Health and Clinical Excellence (NICE)6 and European Society of Cardiology (ESC) guidance7–9 recommend screening patients at risk of CVD based on the multitude of well-documented risk factors for the development of CVD. First established in the original Framingham Heart Study10, many multivariable risk-scoring systems have evolved, with Qrisk11 recommended by NICE6, and the Systematic COronary Risk Evaluation (SCORE12) by ESC7,8. Risk tools incorporate two concepts. Firstly, a combined global risk assessment, where individual risk factors are multiplicative rather than additive, interacting and amplifying harm when present in combination. Secondly, a risk continuum, where risk is not simply present or absent, but rather graded between high and low levels and modifiable.
Risk scores stratify patients into groups based on overall risk of future CV events, with recommendation regarding any interventions (e.g. lifestyle, pharmacotherapy, treatment targets, etc.) dependent on level of risk. Risk stratification as defined by ESC is outlined in Table 1 below, and uses SCORE, a marker of overall 10-year risk of CVD mortality. The ESC recommend that “the approach taken to the prevention of atherosclerotic CVD in a given person should relate to his or her total CV risk: the higher the risk, the more intense the action should be”8. The onus is, therefore, on the clinician to robustly risk stratify each individual patient. Patients with documented CVD will have their CV modifiable risk factors assessed and qualify for pharmacotherapy. Alongside this, both NICE and ESC recognise that patients can be identified as in a high-risk (or above) category based on key co-morbidities, such as type I diabetes mellitus, familial hypercholesterolaemia, or chronic kidney disease (CKD). In these situations there is no role for risk estimation models; all patients qualify for active management of risk factors.
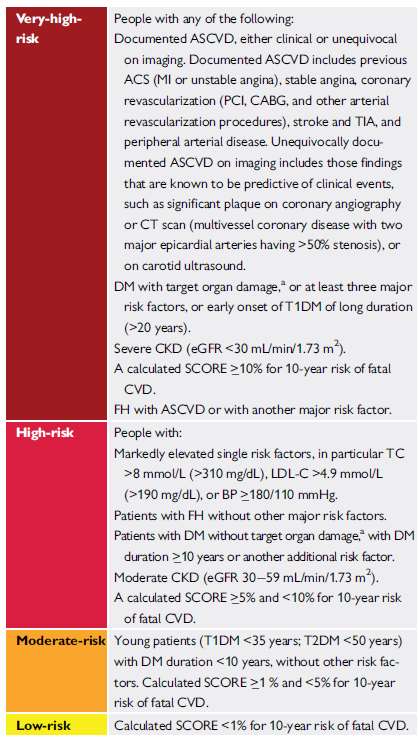
Table 1. Cardiovascular risk categories as outlined by European Society of Cardiology, adapted from Mach et al8 (ASCVD = atherosclerotic cardiovascular disease; ACS = acute coronary syndrome; BP = blood pressure; CABG = coronary artery bypass graft surgery; CKD = chronic kidney disease; CT = computed tomography; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; PCI = percutaneous coronary intervention; SCORE = Systematic Coronary Risk Estimation; T1DM = type 1 DM; T2DM = type 2 DM; TC = total cholesterol; TIA = transient ischaemic attack; a = target organ damage is defined as microalbuminuria, retinopathy, or neuropathy)
Current multivariable probability risk scores have been proven to both over and under treat individuals, and whether they lead to overall benefit in their current form has been called into question13. Their use may commit a large proportion of the middle-aged population to pharmacotherapy, and whether the risk score carries sufficient weight to influence compliance to therapy or recommended lifestyle changes requires further assessment. Risk scoring systems are known to overestimate absolute risk and under appreciate relative risk, whilst the relatively short time-frame (10 year) of risk estimation may underestimate lifetime risk in the young8,13.
Coronary Artery Calcification Score
The coronary artery calcium score (CACS) provides a gross overall assessment of burden of calcification, most commonly using the Agatston score. CACS is robustly demonstrated to provide prognostic value in low and intermediate risk groups14, and to improve net reclassification above traditional risk scores in the assessment of intermediate risk patients(15,16). On this basis, ESC guidance on CVD prevention (20167), dyslipidaemia (20198), and chronic coronary syndrome (20199) all recognised its role in the CVD risk assessment on an individual patient screening basis. They advised that CACS may improve risk classification in asymptomatic patients in the moderate or low risk categories (class IIb level of evidence B recommendation). At levels of risk above this, lifestyle and potential pharmacotherapy is indicated. Therefore, in the absence of symptoms or ECG changes, further imaging (either a non-invasive functional assessment and/or invasive coronary angiography) currently adds little to the intermediate or low risk patient pathway with this approach.
Separate to a dedicated CACS scan, a recent consensus statement from the British Society of Cardiovascular Imaging / British Society of Cardiac CT and British Society of Thoracic Imaging has recommended that the presence of coronary calcification be reported on all CT scans where the heart is within the field of view, regardless of whether the scan is a dedicated, gated cardiac CT or not17. The authors recognised that in the majority these will be asymptomatic patients, therefore are reporting it as an incidental finding, which to a cardiologist could be likened to the reporting of pulmonary nodules on CT coronary angiography (CTCA). They state that coronary artery calcification “is a frequent finding on thoracic CT and is not necessarily an indication for further imaging or referral to a cardiologist. Instead, review of the clinical features and cardiovascular risk factors is recommended, usually by the general practitioner or referring physician”. This provides an opportunity to flag an at-risk patient to the referrer for a CVD review.
Could CTCA improve risk stratification?
As discussed, the detection of coronary calcification carries a significant, and useful, prognostic marker. However, calcification is well described to represent a later stage process in the pathogenesis of atherosclerosis18. As a result, would imaging techniques that detect atherosclerosis at earlier stages of development not be more applicable in a CVD prevention strategy?
CTCA already plays a fundamental role in the assessment of symptomatic, stable chest pain patients. NICE clinical guidance (CG9519) recommends investigation with CTCA for patients presenting with stable chest pain if they have typical or atypical angina, or if they have non-anginal chest pain with ECG changes suggestive of coronary disease.
Importantly, in the CONFIRM registry, CACS demonstrated a significant impact on the restratification of CVD risk, which CTCA did not achieve, when compared with CACS16. However, CTCA techniques have improved dramatically since the early work on prognostication with CACS, and perhaps this comparison would differ now. Along with its well documented impact on changes in the
diagnosis and treatment of symptomatic patients, the 5-year outcome data of the SCOT-HEART I trial demonstrated that the use of CTCA was associated with an improvement in prognosis20. In this study patients assessed in the rapid access chest pain clinic (i.e. symptomatic) underwent CTCA guided assessment or standard care assessment. As well as demonstrating significant re-stratification in the diagnosis of CAD21, CTCA became the first cross-sectional imaging modality to be directly related to a management plan that improved prognosis. Importantly, the study was not designed to assess whether this was due to increased prescription of medical therapy (such as statins or aspirin), or the impact on behavioural change of a patient knowing their diagnosis, or a combination of these factors.
CTCA confers numerous advantages to CACS. First, is to consider whether the presence of coronary calcification alone is sufficient, or whether an assessment that includes the anatomical location, plaque composition and degree of stenosis adds important value. The CADS-RADS criteria have been established as the gold standard for reporting the anatomical assessment of luminal stenosis on CTCA, as demonstrated in Table 222. This has been proven to effectively stratify patients according to risk for adverse events23.
Building on this, a CTCA-based risk score using a combined, weighted assessment of CAD severity based on degree of stenosis, location (weighted relative to left or right dominant circulation) and plaque composition in a segmented model of the coronary tree has been developed24. This compared favourably vs the current anatomical assessment of CTCA based on stenosis only with CAD-RADS, providing better discrimination and reclassification of events.
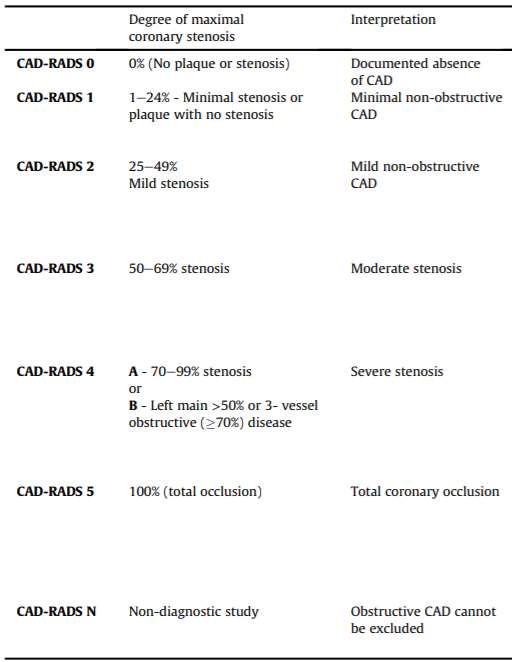
Table 2. CADS-RADS system for reporting anatomical assessment of coronary plaque on CTCA. Adapted from Cury, et al. J Cardiovasc Comput Tomogr. 2016;10(4):269-281; soi:10.1016/j.jcct.2016.04.005, with permission under the terms of the Creative Commons user licence [https://creativecommons.org/licenses/by/4.0/])
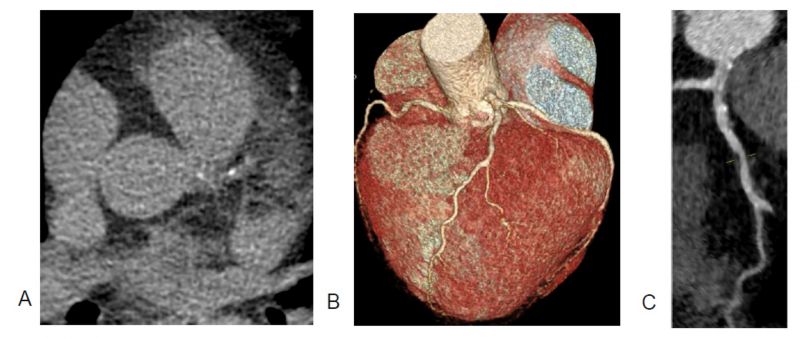
Figure 1. Conflicting results are observed in the same patient: a coronary artery calcium score (CACS) of 11 HU (panel A) and an abnormal CT coronary angiogram (CTCA, panels B and C), both scans taken in the same session. Whilst the CACS demonstrates a low score in the left anterior descending (LAD) artery, the CTCA in fact demonstrated a higher burden of disease, with several LAD plaques of mixed morphology and three high-risk plaque features observed. Panel C highlights a non-calcified plaque at the border of proximal-mid LAD causing mild (25-49%) stenosis, with low-attenuation core, a high-risk plaque feature, whilst further LAD disease demonstrated both positive remodelling and spotty calcification. Image provided with permission by the Royal United Hospitals Bath NHS Foundation Trust database
In addition to degree of luminal stenosis, CTCA offers the opportunity to assess for multiple other markers of additional prognostic value to the pure assessment of CAD presence, not available with CACS25. These include:
- Presence of non-calcific plaque, which is itself associated with a higher risk of future adverse events than calcific disease (see Figure 1). In patients presenting with stable chest pain, lowattenuation plaque burden was recently proven as the strongest predictor of fatal or nonfatal myocardial infarction. These findings challenge the current perception of the supremacy of current classical risk predictors for myocardial infarction, including stenosis severity26.
- Overall plaque burden. Whilst a low CACS has an excellent predictive value for excluding
obstructive CAD(27), the relevance of this finding, particularly in an asymptomatic population is unclear. Recent evidence demonstrated that overall plaque burden, which CTCA provides, rather than obstructive coronary stenosis is a more important prognostic marker28. - Detection of high-risk plaque features, as markers of “vulnerability” based on the plaque composition (e.g. positive remodelling, low attenuation, spotty calcifications), and plaque location, which predict MI risk29.
- FFRCT analysis of coronary flow across a given stenosis now provides the potential for a dual anatomical and physiological assessment with a single test, and the change in FFRCT across a lesion is a proven prognostic marker30.
The incremental value added by this information from a single test may enable the refinement of the risk assessment of the individual patient. However, these findings were derived from studies predominately using symptomatic patients, where CAD was clinically suspected.
Artificial Intelligence and CT Coronary Angiography
In the asymptomatic population, myocardial infarction may be the first presentation of CAD in up to 50-60% of patients without any prior symptoms31. Therefore, it may be reasonable to suggest that any screening tool for CVD prevention should not only seek to identify patients prior to any established CAD leading to a major adverse cardiovascular event (MACE), but also prior to the establishment of significant CAD. Inflammation has long been recognised as a potent driver in the pathogenesis of atherosclerosis32.
Novel CTCA techniques now offer the ability to image coronary inflammation using artificial intelligence (AI) analysis of any standard CTCA using a marker named the pericoronary fat attenuation index (FAI). The research group behind this demonstrated that human vasculature exerts paracrine effects on the surrounding perivascular adipose tissue (PVAT), affecting local intracellular lipid accumulation in pre-adipocytes and creating dynamic changes in the balance between water and lipid content in the presence of inflammation33. A machine-learning algorithm was then trained to analyse the radiomic profile of PVAT, validated against tissue biopsies of vessels taken at time of CABG to identify this fluid gradient on CT images unseen by the human eye34. An example is given in Figure 2. The CRISP-CT study35 then reported on a post-hoc analysis of outcome data gathered prospectively in two independent cohorts of consecutive patients undergoing CTCA. This validated the prognostic value of perivascular FAI for all-cause and cardiac mortality. In doing so, they demonstrated improvement in MACE prediction beyond “traditional risk stratification that included risk factors, coronary calcium score, coronary stenosis, and high-risk plaque features on CCTA”.
A subsequent study advanced this technique further using a process named radiotranscriptomics, incorporating both additional AI analysis and genetic data, and validated its use36. The authors report that “high perivascular FAI values (≥ -70.2 HU) are an indicator of increased cardiac mortality and, therefore, could guide early targeted primary prevention and intensive secondary prevention in patients”.
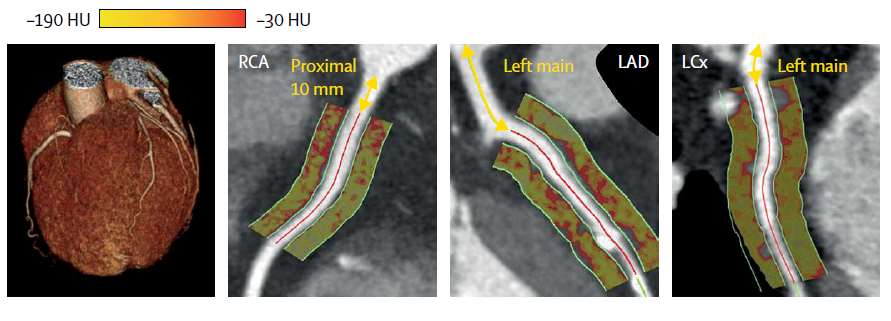 Figure 2. Panels demonstrating the visual appearance of artificial intelligence in assessing the perivascular fat attenuation index (FAI) phenotype of the proximal segments of the three major epicardial coronary arteries, the Hounsfield units analysed correlating to fluid gradients seen in coronary inflammation and corresponding to FAI colour maps. Adapted from: Oikonomou EK, et al.35, with permission under the terms of the Creative Commons user licence [https://creativecommons.org/licenses/by/4.0/].
Figure 2. Panels demonstrating the visual appearance of artificial intelligence in assessing the perivascular fat attenuation index (FAI) phenotype of the proximal segments of the three major epicardial coronary arteries, the Hounsfield units analysed correlating to fluid gradients seen in coronary inflammation and corresponding to FAI colour maps. Adapted from: Oikonomou EK, et al.35, with permission under the terms of the Creative Commons user licence [https://creativecommons.org/licenses/by/4.0/].
CTCA in the asymptomatic population
Ultimately, the use of CTCA in asymptomatic patients is not currently recommended. Indeed, the recent ESC guidelines on chronic coronary syndromes advised that “data demonstrating improved prognosis following appropriate management based on new biomarkers are still lacking”9. They state that in low-risk non-diabetic asymptomatic adults, CTCA (or functional imaging for ischaemia) is not indicated (class III [C] recommendation).
However, CTCA is, in fact, already being used in this group37. In the large CONFIRM registry, which enrolled >25,000 patients across six countries between 2003 and 2009, at least 28% of patients (>7,500) who underwent CTCA were classified as asymptomatic16. These patients all needed to have an indication for CTCA that could include risk assessment of CAD in individuals with history of peripheral arterial disease, cerebrovascular disease, or multiple CAD risk factors, or congenital heart disease. Further, in a 2009 survey of 169 institutions across 38 countries, 34% reported the CTCA indication as “for the exclusion of CAD in clinically healthy patients”38. Of course, it is important to note that, given the multi-national nature of this analysis, practice and incentives may vary significantly to current UK practice. However, one could argue that the NICE recommendation for CTCA in the assessment of patients with nonanginal chest pain if potential important ECG changes are present19 is a screening of a borderline asymptomatic population given the lack of convincing symptoms to support assessment for anything other than prognostic (rather than truly symptom driven) benefit. Interestingly, the SCOTHEART investigators reported that in the 5-year outcome follow-up data, the most significant relative reduction in coronary events was observed in those with non-anginal chest pain irrespective of their cardiovascular risk score39.
The Intervention:
The Wilson and Jungner criteria outline the importance of “an accepted treatment for patients with recognised disease”. In the case of early CAD, relatively cheap, effective and proven interventions that are simple to institute are well established. This can include behavioural intervention, with advice on lifestyle factors, review and optimisation of important co-morbidities (e.g. hypertension and diabetes), or consideration of well-established medications such as statin therapy, or (more contentiously) aspirin. In the presence of CTCA proven CAD, the institution of these interventions would be supported with evidence of underlying pathology that may in itself have added benefit to patient behaviours and compliance, though this requires further assessment.
Potential drawbacks
The recommendation of CTCA for any patient (regardless of symptoms) in whom optimal medical therapy for CAD is not already indicated would be a significant sea change. The cost implication to the healthcare system is important, and will need weighing against the overall current impact of CVD on patients and the health economy. As described, the ESC have clearly stated in recent guidance they feel data demonstrating improved prognosis (in the asymptomatic group) following appropriate management based on these new biomarkers are needed first9.
Specifics to the CT scan and service delivery require consideration. The ongoing work to reduce radiation exposure is important, as current CTCA techniques remain at or just above the equivalent dose to 1-years background radiation exposure in the UK (estimated 2–5 mSv for CTCA (40) vs ~2.7mSv background radiation41). For context, exposure to 100mSv is believed to increase lifetime cancer risk by 0.5%, however this is extrapolated from large studies based on uranium miners and atomic bomb survivors42. Extrapolation back from this estimate is not recommended based on confounding factors, and no studies have directly assessed the impact of low doses of radiation (as used in the majority of radiology) in adults, so it is unclear what the true impact of a single 1 mSv scan is.
The additional risk of a higher radiation dose associated with CTCA vs CACS has also significantly reduced with modern scanners and acquisition techniques40, and in many cases they are now on a par. As a result, this is now less of a consideration in a direct comparison between fulldose CACS and CTCA, where now the relative merits of the two should be considered in more detail.
Contrast reaction rates are very low, particularly for severe adverse reactions43. However, the importance of this increases with a population-based approach in asymptomatic individuals where the risk-benefit ratio differs from that of symptomatic patients, particularly where the index of suspicion is lower. Availability of and expertise in reporting CTCA has increased significantly since its recommendation for a large proportion of chest pain patients in NICE CG9519. If widespread use in asymptomatic patients were to be recommended, this would likely require even more investment. The same may also be true of potential incidental findings, which may require more face-to-face appointments to discuss results, and/or further surveillance imaging, which may add cost and workload to the system. This may be politically untenable in the current climate given the increase in waiting times brought on by the covid-19 pandemic, however, this should not alter the crucial long-term goal of an optimal CVD prevention strategy.
The importance of not falsely reassuring individuals with no CAD on CTCA is important, particularly young patients where lifetime CV risk may still be above normal. For example, it is well recognised that young patients with genetic dyslipidaemias may have no contemporaneous CT evidence of CAD but remain at significant risk of future MACE8. Therefore, patient selection for use of CTCA as a screening tool is important. Indeed, there are other groups where CTCA is less helpful or contraindicated. These include, but are not limited to, those with extensive coronary calcification (blooming artefact affects quality of vessel analysis) and significant chronic kidney disease (though these patients are already “high-risk” and so qualify for primary prevention strategies). In others, quality of imaging may be impaired, such as in morbid obesity or atrial fibrillation (AF). In the case of AF, however, imaging techniques are advancing with flash scan technology enabling the acquisition of diagnostic data in a single heartbeat, which can negate the impact of the dysrhythmia.
Lastly, the possible inclination to further assess asymptomatic patients with “significant” CAD (e.g. a proximal LAD lesion) identified on a screening CTCA for potential “prognostic benefit” would need careful consideration. The role of revascularisation in the symptomatic, stable angina population is summarised elsewhere44, and has recently been informed by the ISCHAEMIA trial45, although this did not recruit patients with left main disease, and continues to have the potential to expose an asymptomatic population to more than they bargained for.
The Future
In keeping with the ESC highlighted lack of evidence for the impact of CTCA on patient outcomes in asymptomatic populations, the SCOTHEART II trial46, currently recruiting, will hopefully contribute significantly to this area. This will provide a direct comparison between CTCA and a validated CV risk score (ASSIGN Cardiovascular Risk Score, used routinely in Scotland) in 6,000 middle-aged individuals at risk of CVD. It should help to clarify whether the clear benefit of optimal medical therapy in symptomatic populations is shared by those who are asymptomatic.
Where this fits into the growing potential around AI techniques to assess coronary inflammation discussed earlier remains to be seen. The Oxford Risk Factors And Non Invasive Imaging Study (ORFAN) is also currently recruiting 8,500 adult participants where a CTCA has been requested by the clinical team (therefore may include a higher volume of symptomatic patients), alongside a blood sample. With this, they will assess a person’s likelihood of having a cardiovascular event such as a heart attack or stroke in the future by crosslinking the information gathered from CT images of adipose tissue and biomarkers in the blood. They are also retrospectively collecting 10,000 CT scans in order to develop new AI tools to allow automation of the image analysis processes47.
Lastly, low-dose CACS (equivalent to as low as ~0.01mSv) has also been validated against full-dose CACS48, which may provide an even lower risk study (based on absence of contrast, and reduced radiation dose), and this may need comparison to both an AI assessment of coronary inflammation, or standard CTCA, in the asymptomatic population.
Conclusion
Despite advancements in treatments, technology, and current CVD prevention strategies the fact that CAD remains a leading cause of morbidity and mortality in the UK would suggest a significant role for improving the screening and primary prevention of CVD in the NHS. Based on ESC guidance it may be considered (class IIb [B]) to image an asymptomatic patient to assist with CV risk screening – though currently this would be with coronary artery calcium scoring rather than formal CT coronary angiography, which is rated as not recommended (class III [C]) in this cohort. Its wider use on a population basis is not currently recommended in UK guidance, however, the increasing evidence-base for cardiac CT techniques in the symptomatic population suggests it may prove instrumental to optimising individual patient risk assessment and upcoming trials and evolution in technology may well change this.
The question remains, however, if asked to perform a comprehensive cardiovascular risk assessment of an asymptomatic patient, particularly in a low or moderate risk group, what would you do?
Disclosures
None
References
- British Heart Foundation. UK Factsheet. Br Hear Found. 2020;(January):1–21.
- NHS. The NHS long term plan [Internet]. 2019. Available from: https://www.longtermplan.nhs.uk/publication/nhs-longterm-plan/
- Wilson, James Maxwell Glover, Jungner G. Principles and practice of screening for disease. World Health Organization. 1968.
- Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: A review of
screening criteria over the past 40 years. Bull World Health Organ. 2008;86(4):317–9. - Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in Cardiovascular Mortality:
Possible Causes and Implications. Circ Res. 2017;120(2):366–80. - NICE. Cardiovascular disease: risk assessment and reduction, including lipid modification. Clin Guidel [CG181].
2016;(September 2016). - Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on
cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37(29):2315–81. - Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the
management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. - Knuuti J, Wijns W, Achenbach S, Agewall S, Barbato E, Bax JJ, et al. 2019 ESC guidelines for the diagnosis and
management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. - Mahmood, Syed S, Levy, Daniel, Vasan, Ramachandran S, Wang TJ. The Framingham Heart Study and the Epidemiology of Cardiovascular Diseases: A Historical Perspective. Lancet. 2014;383(9921):999–1008.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: Prospective open cohort study. Br Med J. 2007;335(7611):136–41.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal
cardiovascular disease in Europe: The SCORE project. Eur Heart J. 2003;24(11):987–1003. - Studziński K, Tomasik T, Krzysztoń J, Jóźwiak J, Windak A. Effect of using cardiovascular risk scoring in routine risk
assessment in primary prevention of cardiovascular disease: An overview of systematic reviews. BMC Cardiovasc Disord. 2019;19(1):1–16. - Kelkar AA, Schultz WM, Khosa F, Schulman-Marcus J, O’Hartaigh BWJ, Gransar H, et al. Long-Term Prognosis after Coronary Artery Calcium Scoring among Low-Intermediate Risk Women and Men. Circ Cardiovasc Imaging. 2016;9(4):1–7.
- Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of Novel Risk Markers for Improvement in Cardiovascular Risk Assessment in Intermediate Risk Individuals. The Multi-Ethnic Study of Atherosclerosis. Jama. 2014;308(8):788–95.
- Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM registry (Coronary CT angiography evaluation for clinical outcomes: An international mult. Circulation. 2012;126(3):304–13.
- Williams MC, Abbas A, Tirr E, Alam S, Nicol E, Shambrook J, et al. Reporting incidental coronary, aortic valve
and cardiac calcification on non-gated thoracic computed tomography, a consensus statement from the BSCI/BSCCT and BSTI. Br J Radiol. 2020;(July):20200894. - McEvoy JW, Blaha MJ, DeFilippis AP, Budoff MJ, Nasir K, Blumenthal RS, et al. Coronary artery calcium progression: An important clinical measurement? J Am Coll Cardiol. 2010;56(20):1613–22.
- NICE. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Clin Guidel [CG95].
2016;(November 2016). - Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–33.
- Newby D, Williams M, Hunter A, Pawade T, Shah A, Flapan A, et al. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOTHEART): An open-label, parallel-group, multicentre trial. Lancet [Internet]. 2015;385(9985):2383–91. Available from: http://dx.doi.org/10.1016/S0140-6736(15)60291-4
- Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, et al. Coronary Artery Disease - Reporting and Data System (CAD-RADS): An Expert Consensus Document of SCCT, ACR and NASCI: Endorsed by the ACC. JACC Cardiovasc Imaging. 2016;9(9):1099–113.
- Xie JX, Cury RC, Leipsic J, Crim MT, Berman DS, Gransar H, et al. The Coronary Artery Disease–Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated With Standardized Coronary Computed Tomography Angiography Reporting. JACC Cardiovasc Imaging. 2018;11(1):78–89.
- van Rosendael AR, Shaw LJ, Xie JX, Dimitriu-Leen AC, Smit JM, Scholte AJ, et al. Superior Risk Stratification With Coronary Computed Tomography Angiography Using a Comprehensive Atherosclerotic Risk Score. JACC Cardiovasc Imaging [Internet]. 2019;12(10):1987–97. Available from: http://dx.doi.org/10.1016/j.jcmg.2018.10.024
- Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn A V., Khamis RY, et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(10):1226–43.
- Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-Attenuation Noncalcified
Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results from the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation. 2020;1452–62. - Abdool MA, Ashrafi R, Davies M, Raga S, Lewis-Jones H, Thwaite E, et al. A UK cardiac centre experience of low-risk, stable chest pain patients with calcium score of zero. Br J Cardiol. 2014;21(2):78.
- Mortensen MB, Dzaye O, Steffensen FH, Bøtker HE, Jensen JM, Rønnow Sand NP, Kragholm KH, Sørensen HT,
Leipsic J, Mæng M, Blaha MJ NB. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J Am Coll Cardiol. 2020;76(24):2803–13. - Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive Assessment of Plaque
Morphology and Composition in Culprit and Stable Lesions in Acute Coronary Syndrome and Stable Lesions in Stable Angina by Multidetector Computed Tomography. J Am Coll Cardiol. 2006;47(8):1655–62. - Lee JM, Choi G, Koo BK, Hwang D, Park J, Zhang J, et al. Identification of High-Risk Plaques Destined to Cause Acute Coronary Syndrome Using Coronary Computed Tomographic Angiography and Computational Fluid Dynamics. JACC Cardiovasc Imaging. 2019;12(6):1032–43.
- Mittal TK, Barbir M, Rubens M. Role of computed tomography in risk assessment for coronary heart disease.
Postgrad Med J. 2006;82(972):664–71. - Peter Libby. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74.
- Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: The regulatory role of perivascular adipose tissue. Diabetes. 2015;64(6):2207–19.
- Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary
inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398). - Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet [Internet]. 2018;392(10151):929–39. Available from: http://dx.doi.org/10.1016/S0140-6736(18)31114-0
- Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, et al. A novel machine learning derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CTangiography. Eur Heart J. 2019;40(43):3529–43.
- Meinel FG, Renker M. Coronary CT Angiography for Screening, Risk Stratification, and Management of
Asymptomatic Patients: State of the Evidence. In: CT of the Heart. Humana, Totowa, NJ; 2019. p. 739–45. - Maurer, MH, Zimmermann, E, Schlattmann, P, Germershausen, C, Hamm, B, Dewey M. Indications, imaging
technique, and reading of cardiac computed tomography: survey of clinical practice. Eur Radiol. 2012;22:59–72. - Williams MC, Moss A, Nicol E, Newby DE. Cardiac CT Improves Outcomes in Stable Coronary Heart Disease: Results of Recent Clinical Trials. Curr Cardiovasc Imaging Rep. 2017;10(5):1–6.
- Williams MC, Stewart C, Weir NW, Newby DE. Using radiation safely in cardiology: What imagers need to know.
Heart. 2019;105(10):798–806. - Public Health England. Guidance Ionising radiation: dose comparisons [Internet]. 2011. Available from:
https://www.gov.uk/government/publications/ionising-radiation-dose-comparisons/ionising-radiation-dose-comparisons#:~:text=The 2.7 mSv dose that,radiation in homes and workplaces. - Sobue T. Scientific approach to radiation-induced cancer risk. Fukushima J Med Sci. 2011;57(2):90–2.
- Katayama, H, Yamaguchi, K, Kozuka, T, Takashima, T, Seez, P, Matsuura K. Adverse reactions to ionic and nonionic
contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621–8. - Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines
on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. - David J. Maron, M.D., Judith S. Hochman, M.D., Harmony R. Reynolds, M.D., Sripal Bangalore, M.D., M.H.A., Sean M. O’Brien, Ph.D., William E. Boden, M.D., Bernard R. Chaitman, M.D., Roxy Senior, M.D., D.M., Jose López- Sendón, M.D., Karen P. Alexander, M.D. PD. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382(15):1395–407.
- Newby DE. Computed Tomography Coronary Angiography for the Prevention of Myocardial Infarction (The SCOTHEART 2 Trial) (SCOT-HEART 2) [Internet]. 2019 [cited 2020 Dec 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT03920176
- Antoniades, Charalambos, Channon, Keith M, Antonopoulos, Alexios, Thomas, Sheena, Lyasheva, Maria, Oikonomou, Evangelos, Sabharwal, Nikant, Shirodaria, Cheerag, Uberoi, Raman, Anthony, Susan, Banning, Adrian, Kelion, Andrew, Kardos, Attila, Nicol, Ed, Adl S. The Oxford Cohort for Heart, Vessels & Fat [Internet]. 2019. Available from: https://oxhvf.com/the-orfan-study/
- Vingiani V, Abadia AF, Schoepf UJ, Fischer AM. Low-kV coronary artery calcium scoring with tin filtration using a kVindependent reconstruction algorithm. J Cardiovasc Comput Tomogr. 2020;14(3):246–50.
