Refractory VT – Can we noninvasively re-programme the heart?
| Take Home Messages |
|---|
|
Introduction
Sudden cardiac death (SCD) causes circa 20% of deaths in Europe1. The majority occurs secondary to malignant ventricular arrhythmia in patients with ischaemic heart disease2,3. SCD also accounts for 30-50% of deaths in patients with heart failure4, and given the increasing prevalence of HF5, preventing SCD in this cohort is a growing clinical need.
Current Strategies for Treating Malignant Arrhythmia
Anti-arrhythmic drugs have significant side effects and limited efficacy6. Implantable cardioverter defibrillators improve survival in patients at risk of SCD, however, this comes at the cost of reduced quality of life from shocks7 and device complications.
Radiofrequency catheter ablation (CA) is recommended first line in patients with treatment refractory scar related VT8. However, it is time consuming, technically challenging and operator dependent. It carries an 8-10% overall complication risk, including stroke, tamponade, AV block and valve damage9. Rates of VT cessation are high (77.4% non-inducibility of VT) in newcomers with focal scar10 but are disappointing in patients with severe ventricular impairment, diffuse scar11 or following re-do procedures.
New Developments
Recent advances in stereotactic body radiation therapy (SBRT) may be the much-awaited solution to successfully treating ventricular arrythmia in a non-invasive manner.
SBRT is a focused radiation treatment that is well established in oncology where it is used to achieve high rates of tumour control with minimal damage to adjacent tissues12.
Early studies focussed on the creation of fibrotic lesions to interrupt action potential conduction within six months of RT13,14. However, several studies have demonstrated a significant reduction in VT burden with RT treatment much earlier than this - The Cuculich group demonstrated a 99.9%reduction in VT burden in five patients with high-risk VT refractory to catheter ablation within just four weeks of RT treatment15.
More recently, the ENCORE-VT trial16 investigated the effect of a single fraction of 25Gy radiation in 19 patients. The frequency of VT episodes was reduced by 75% in 89% of patients within six weeks of treatment. Again, this is well before replacement fibrosis would be expected -estimated to take over 70 days17,18. In addition, early pre-clinical studies investigating cardiac RT demonstrated that whilst doses of 40 to 160Gy were sufficient to induce cardiac fibrosis, lower doses as used in the ENCORE-VT trial were not19.
Therefore, the question of how RT at 25Gy reduces ventricular tachycardia remains unanswered.
In a quest for the answer, this editorial outlines a fascinating piece from Rentschler’s group20 –taking us from the laboratory bench to the bedside, in a move to define the effects of single-fraction stereotactic radiotherapy on the heart. But first, it is useful to revisit the physiology of re-entrant arrhythmia.
Electrophysiology
Re-entry remains the most common mechanism of VT - a result of heterogenous conduction through the myocardium, typically secondary to ischaemia related scar.
Concept 1
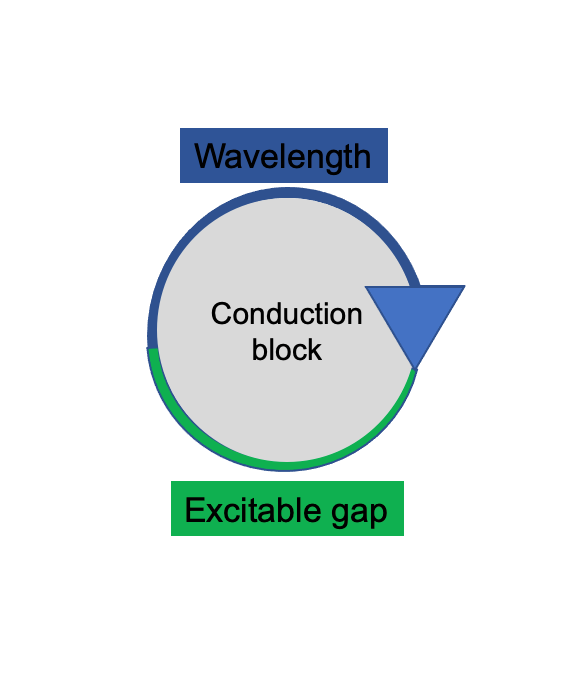
To produce sustained re-entry, the conduction wavelength – the distance travelled by an impulse while the myocardium is refractory – must be shorter than the path of conduction. This is required to maintain an excitable gap (Figure 1). Therefore, the longer the wavelength, the less likely re-entry is to be sustained. Anti-arrhythmic therapy can therefore function by increasing the wavelength.
Concept 2
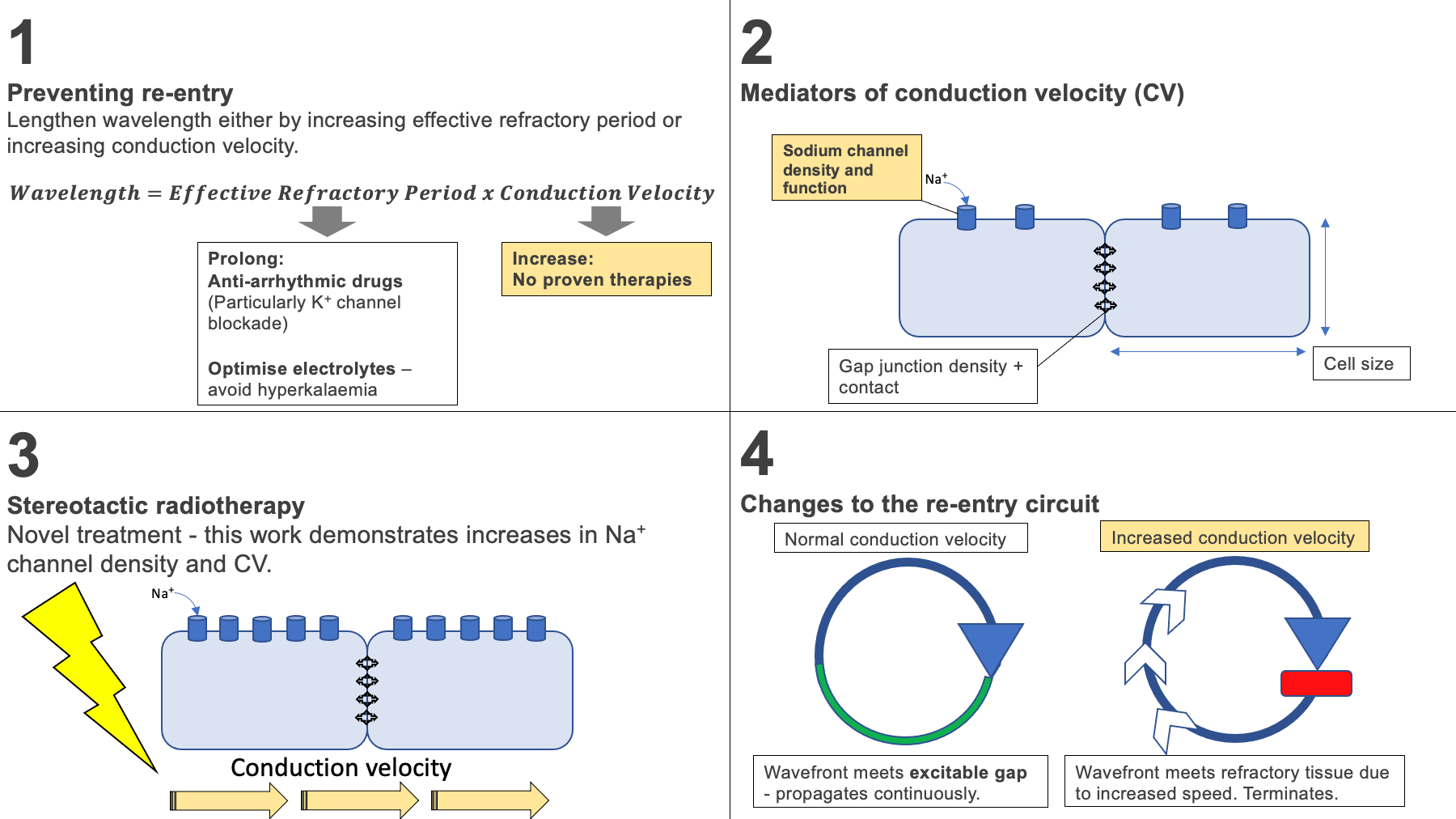
The conduction wavelength is equal to the product of the effective refractory period (ERP) - the amount of time in which the cell cannot respond to a new stimulus - and the conduction velocity (CV). Lengthening the wavelength by either increasing the ERP or CV reduces the likelihood of re-entry. Anti-arrhythmic drugs can lengthen the ERP, however there are currently no proven therapies which increase CV.
Wavelength = Effective refractory period x conduction velocity
Figure 1. Demonstration of requirement for a short wavelength to sustain re-entry. The longer the wavelength, the less likely the wavefront is to meet excitable tissue.
So how can we increase conduction velocity?
In this eminent paper20, the group demonstrate that 25Gy cardiac RT does not induce cardiac fibrosis within the time-frame of VT reduction, which had been the suggested mode of effect. Instead, they demonstrate that RT leads to increased conduction velocity and genetic modulation within the mammalian heart.
Important Results:
Result 1 - Twenty-five Gy radiation does not increase cardiac fibrosis in patients despite marked decreases in VT.
The relationship between 25Gy radiation and cardiac fibrosis was investigated using four VT patients who received cardiac RT and provided post-mortem specimens. Targeted regions were compared to non-targeted regions within the same hearts.
Only minor differences were observed between targeted and non-targeted myocardium, yet within 4 to 6 weeks all four patients experienced substantial reductions in VT, with no sustained episodes on their ICDs.
Result 2 - Twenty-five Gy radiation increases conduction velocity.
Using a murine model, the effects of 25Gy RT on cardiomyocytes was further investigated at six weeks post RT. As demonstrated in the human myocardium, there was no evidence of fibrosis. Interestingly, a significantly shortened QRS was observed compared to controls, whilst other ECG variables were unchanged. Ventricular conduction velocity was also significantly increased (by 29%) when compared to controls without a change in repolarisation. This suggests RT increases conduction velocity, thus lengthening the conduction wavelength and preventing re-entry, as outlined in concept 2.
Result 3 – RT increases levels of conduction proteins.
The major determinants of CV are cell size, sodium channel density and gap junctions. There was no difference in cell size between the two groups. However, there was an 80% increase in the density of NaV1.5 - the pore forming subunit of the voltage-gated sodium channel responsible for phase 0 of the action potential – when compared to controls. These mechanisms may therefore explain how RT increases CV and prevents re-entry (Figure 2).
Result 4 – These changes persist beyond the short-term.
Phase 2 trials have demonstrated a persistent 78%reduction in VT following a single fraction of 25Gy RT at two years post treatment16 –suggesting changes induced by RT persist for a clinically relevant period. In mice, the investigators demonstrate persistent elevations in NaV1.5 (70% increase) at 42 weeks post RT. This translated into a persistently increased conduction velocity and shortened QRS.
Result 5 – Radiation increases CV in myocardium that borders scar tissue.
To study the effects of RT in the clinically applicable setting of ventricular scar tissue, the investigators used a murine model of apical infarction. They compared CVs in mice with ligated left anterior descending arteries. Two weeks post MI, mice received 25Gy RT. Border zone myocardium – the area between healthy tissue and scar – was stimulated and CVs were found to be significantly faster in RT mice. Indeed, this was correlated with significantly increased expression of NaV1.5 in border zone myocardium of RT mice compared to controls.
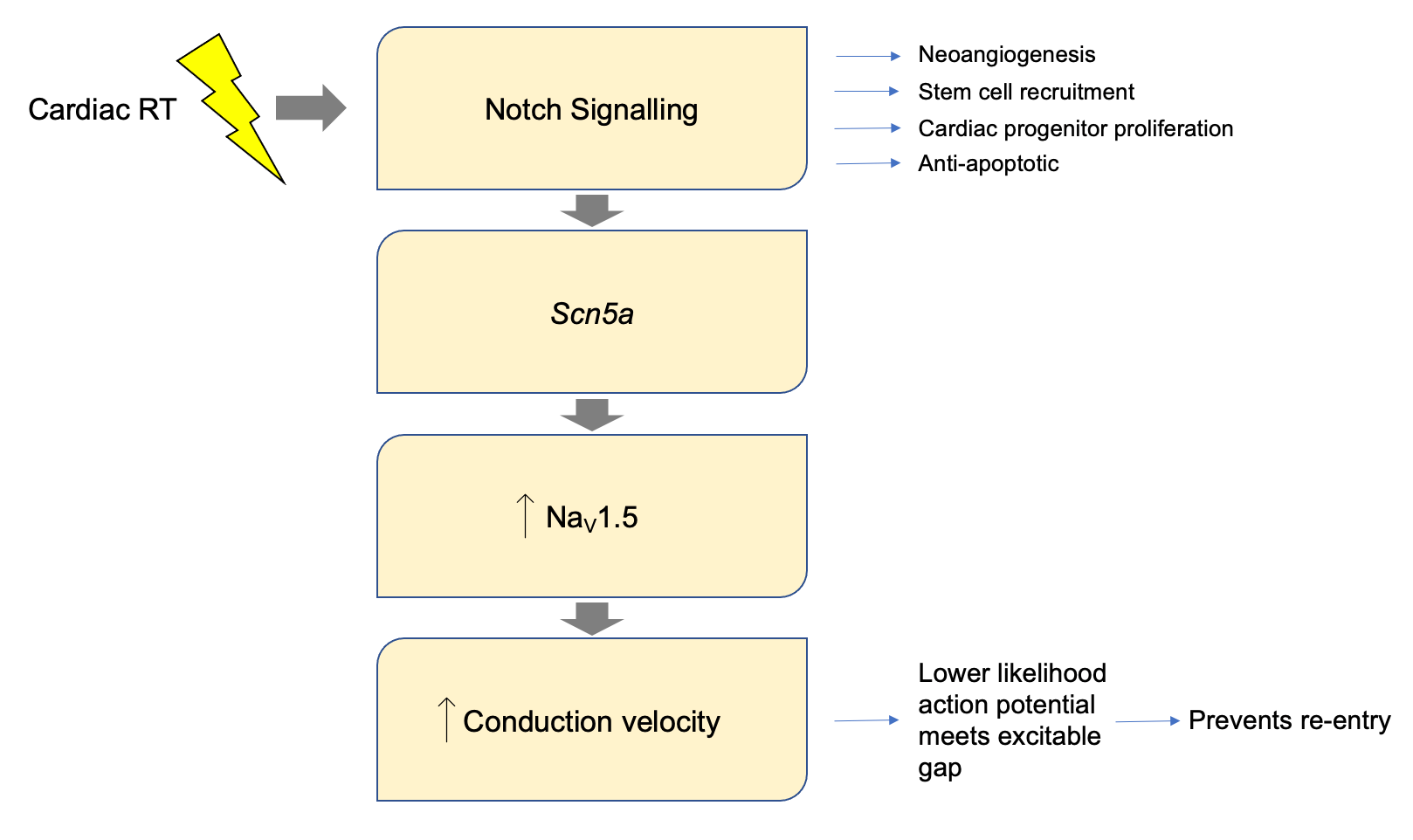
Result 6 – Notch signalling is at least partially responsible for the changes in ion channel expression.
The murine ventricular transcriptome was profiled at baseline and six weeks post RT. Overall, 509 genes were differentially expressed. The Notch pathway, a driver of conduction system development21 and not active in adult cardiomyocytes, was found to be significantly activated following RT. This is important as transient notch reactivation has been linked with up-regulation of NaV1.521,22. Indeed, the investigators showed that in mice where the Notch pathway was transiently activated, there were significant increases in NaV1.5 channel density and CV after 16 weeks compared to controls (Figure 3). Knock out of the Notch pathway followed by RT resulted in a >30% attenuation in CV increase, with a significant reduction in NaV1.5 expression. This suggests that transient notch activation may contribute to RT induced increases in NaV1.5 density, a plausible mechanism for increases in conduction velocity.
Figure 2. Suggested mode of action of cardiac radiotherapy (RT). 1. Increase in conduction velocity increases the conduction wavelength, preventing re-entry. 2. The main mediators of conduction velocity. 3. RT is thought to increase the density of Na+ channels, increasing conduction velocity. 4. This results in a lower likelihood of the wavefront meeting excitable tissue – reducing the likelihood of wavefront propagation and re-entry.
Figure 3. Proposed pathway linking cardiac radiotherapy (RT) to increases in conduction velocity. The Notch pathway plays a key role in conduction system development and is not active in adult cardiomyocytes. Notch was found to be significantly activated post RT. Notch activation is known to up-regulate Scn5a which encodes the NaV1.5 channel. Notch was transiently and selectively activated in cardiomyocytes, resulting in a persistent increase in NaV1.5 density and conduction velocity.
Translating This Data
The authors went on to study electrical properties of an explanted human heart of a patient with non-ischaemic cardiomyopathy previously treated with 25Gy RT. They compared levels of NaV1.5 in the targeted region of the heart with a non-targeted remote region of the same failing heart, which was explanted over two and a half years after cardiac RT.
Compared to two controls, the density of NaV1.5 was much lower in the non-targeted region of the failing heart, consistent with known reductions in the setting of HF23. Over two years following RT, there was a threefold increase in NaV1.5 in the targeted myocardium compared to the non-targeted myocardium. This restored levels of NaV1.5 to those of normal hearts.
Surface ECGs were also analysed for 19 patients who received RT. There was a trend toward QRS shortening six weeks post RT (medians 149ms pre-RT, 139ms post RT). One patient’s ECG changed from left bundle branch block with a QRS of 165ms to a QRS of 130ms without left bundle branch block. This remained for at least six months and may well be a manifestation of RT induced electrical reprogramming in humans.
Discussion Points
Whilst this work provides insights into the effect of RT on the myocardium, as the authors acknowledge, this work does not fully explain the changes in CV and there are several limitations.
Firstly, the translation of data derived from the murine model used to human cardiac electrophysiology is debatable. For example, there was an increase in connexin 43 (a component of the cellular gap junction) in mice following RT, but there was no significant change in connexin 43 levels post RT in the human heart. In addition, the authors waited just two weeks post LAD ligation in their murine model of myocardial infarction (MI) before analysis, much less than the window typically used before re-assessment of ventricular function post MI in humans.
Secondly, adverse effects of RT need consideration. Data from cancer survivors have demonstrated side effects such as pericardial fibrosis, coronary and valve disease. The data from cardiac RT is very limited and makes risk quantification difficult, though studies have reported cases of pericarditis, delayed pericardial effusion and one case of gastro-pericardial fistula two years after treatment24. It is therefore vital that all centres report their experiences going forward. Perhaps one method of ameliorating the side effect profile is to use lower doses of RT, thus future work looking to confirm the minimal effective dose would be useful.
Alternative methods of increasing CV should be considered. In vitro delivery of the skeletal muscle Na+ channel to murine myocardium with an increase in conduction velocity has been demonstrated26, whilst others have trialled murine gene therapy – introducing Cx43 via adenoviruses27. As we progress toward cardiogenomics becoming widely available28, it is conceivable that genomic therapies could be used to moderate conduction velocity – preventing re-entry.
Initial UK Experience
The first British case series demonstrating SBRT in seven patients with refractory VT was published in November 202125. There was a reduction in VT burden of 85% and clinicians were able to down-titrate or cease amiodarone. There was no significant toxicity following RT aside from grade 1 fatigue in two patients. No change in LV function was seen on echo at six weeks. Total ICD shocks were reduced from 7 pre-treatment to none post treatment. One patient had an acute flare of VT that required dose escalation of amiodarone. Two patients died of progressive heart failure. On post-mortem examination, there were no RT related changes in surrounding organs.
Broader Context and future direction
As survival following MI improves, the incidence of VT is likely to increase. It is therefore important that we establish safe, rapid, and affordable means of treatment. RT presents an exciting opportunity that may mitigate the need for drug therapy, implantable cardiac devices, and catheter ablation. This is especially significant in view of the increased risk of catheter ablation in frail patients29 in the setting of an ageing population.
The capacity of the UK radiotherapy service should also be explored – particularly during recovery from the COVID-19 pandemic. If this were to become routine therapy, the cardiology community will benefit from the expert input of our clinical oncology colleagues in service development and treatment planning.
Whilst still in experimental development and lacking long-term safety data, there is an established national multidisciplinary group (UK SABR Consortium) that provides expert consensus, quality assurance and liaison with clinical commissioners to further develop SBRT in the UK.
Lastly, there are four significant areas of inquiry going forward – many under current active investigation.
- Limited data in the field concerning RT for human non-ischaemic re-entrant VT.
- Prolonged survival following RT results in limited post-mortem sample availability–reinforcing the need for large animal, appropriately powered preclinical studies to further elucidate mechanisms.
- Minimal effective dose trials in humans given the side effect profile of 25Gy RT in these small cohorts.
- Larger cohorts with robust long term safety data.
Disclosures
Nil.
References
- ESCAPE-NET [Internet]. [cited 2021 Nov 29]. Available from: https://www.escardio.org/Sub-specialty-communities/European-Heart-Rhythm-Association-(EHRA)/Research-and-Publications/escape-net
- Yow AG, Rajasurya V, Sharma S. Sudden Cardiac Death. StatPearls [Internet]. 2021 Aug 12 [cited 2021 Nov 29]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK507854/
- Centers for Disease Control and Prevention (CDC). State-specific mortality from stroke and distribution of place of death--United States, 1999. MMWR Morb Mortal Wkly Rep [Internet]. 2002 May 24 [cited 2021 Nov 29];51(20):429–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12056498
- Narang R, Cleland JGF, Erhardt L, Ball SG, Coats AJS, Cowley AJ, et al. Mode of death in chronic heart failure. A request and proposition for more accurate classification. Eur Heart J [Internet]. 1996 [cited 2021 Nov 29];17(9):1390–403. Available from: https://pubmed.ncbi.nlm.nih.gov/8880025/
- Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev [Internet]. 2017 [cited 2021 Nov 29];3(1):7. Available from: /pmc/articles/PMC5494150/
- Santangeli P, Muser D, Maeda S, Filtz A, Zado ES, Frankel DS, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Hear Rhythm [Internet]. 2016 Jul 1 [cited 2021 Dec 16];13(7):1552–9. Available from: https://pubmed.ncbi.nlm.nih.gov/26961297/
- Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, et al. Quality of Life with Defibrillator Therapy or Amiodarone in Heart Failure. N Engl J Med [Internet]. 2008 Sep 4 [cited 2021 Nov 30];359(10):999–1008. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa0706719
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Bloma N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac deathThe Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J [Internet]. 2015 Nov 1 [cited 2021 Nov 30];36(41):2793–867. Available from: https://academic.oup.com/eurheartj/article/36/41/2793/2293363
- Pothineni NV, Deshmukh A, Padmanabhan D, Kovelamudi S, Patel NJ, Badheka AO, et al. Complication rates of ventricular tachycardia ablation: Comparison of safety outcomes derived from administrative databases and clinical trials. Int J Cardiol [Internet]. 2015;201:529–31. Available from: http://dx.doi.org/10.1016/j.ijcard.2015.08.116
- Dinov B, Fiedler L, Schönbauer R, Bollmann A, Rolf S, Piorkowski C, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation [Internet]. 2014 Feb 18 [cited 2021 Nov 30];129(7):728–36. Available from: https://pubmed.ncbi.nlm.nih.gov/24211823/
- Tilz RR, Lin T, Eckardt L, Deneke T, Andresen D, Wieneke H, et al. Ablation Outcomes and Predictors of Mortality Following Catheter Ablation for Ventricular Tachycardia: Data From the German Multicenter Ablation Registry. J Am Heart Assoc [Internet]. 2018 Mar 23 [cited 2021 Dec 14];7(6). Available from: http://ahajournals.org
- Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys [Internet]. 2010 [cited 2021 Nov 30];37(8):4078–101. Available from: https://pubmed.ncbi.nlm.nih.gov/20879569/
- Lehmann HI, Graeff C, Simoniello P, Constantinescu A, Takami M, Lugenbiel P, et al. Feasibility Study on Cardiac Arrhythmia Ablation Using High-Energy Heavy Ion Beams. Sci Reports 2016 61 [Internet]. 2016 Dec 20 [cited 2021 Dec 2];6(1):1–13. Available from: https://www.nature.com/articles/srep38895
- Rapp F, Simoniello P, Wiedemann J, Bahrami K, Grünebaum V, Ktitareva S, et al. Biological Cardiac Tissue Effects of High-Energy Heavy Ions – Investigation for Myocardial Ablation. Sci Reports 2019 91 [Internet]. 2019 Mar 21 [cited 2021 Dec 2];9(1):1–13. Available from: https://www.nature.com/articles/s41598-019-41314-x
- Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med [Internet]. 2017 Dec 14 [cited 2021 Dec 2];377(24):2325–36. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1613773
- Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation [Internet]. 2019 Jan 15 [cited 2021 Dec 2];139(3):313–21. Available from: https://pubmed.ncbi.nlm.nih.gov/30586734/
- Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation [Internet]. 2019 Jan 15 [cited 2021 Dec 11];139(3):313–21. Available from: https://pubmed.ncbi.nlm.nih.gov/30586734/
- Ma CX, Zhao XK, Li YD. New therapeutic insights into radiation-induced myocardial fibrosis. Ther Adv Chronic Dis [Internet]. 2019 [cited 2021 Dec 2];10:1–10. Available from: https://pubmed.ncbi.nlm.nih.gov/31448071/
- Lehmann HI, Richter D, Prokesch H, Graeff C, Prall M, Simoniello P, et al. Atrioventricular node ablation in langendorff-perfused porcine hearts using carbon ion particle therapy. Circ Arrhythmia Electrophysiol [Internet]. 2015 Apr 20 [cited 2021 Dec 2];8(2):429–38. Available from: https://www.ahajournals.org/doi/abs/10.1161/circep.114.002436
- Zhang DM, Navara R, Yin T, Szymanski J, Goldsztejn U, Kenkel C, et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun 2021 121 [Internet]. 2021 Sep 24 [cited 2021 Dec 2];12(1):1–14. Available from: https://www.nature.com/articles/s41467-021-25730-0
- Rentschler S, Yen AH, Lu J, Petrenko NB, Lu MM, Manderfield LJ, et al. Myocardial Notch Signaling Reprograms Cardiomyocytes to a Conduction-Like Phenotype. Circulation [Internet]. 2012 Aug 21 [cited 2021 Dec 11];126(9):1058. Available from: /pmc/articles/PMC3607542/
- Khandekar A, Springer S, Wang W, Hicks S, Weinheimer C, Diaz-Trelles R, et al. Notch-Mediated Epigenetic Regulation of Voltage-Gated Potassium Currents. Circ Res [Internet]. 2016 Dec 9 [cited 2021 Dec 11];119(12):1324–38. Available from: https://pubmed.ncbi.nlm.nih.gov/27697822/
- Van Der Zwaag PA, Van Rijsingen IAW, Asimaki A, Jongbloed JDH, Van Veldhuisen DJ, Wiesfeld ACP, et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail [Internet]. 2012 Nov [cited 2021 Dec 11], 14(11):1199-207. Available from: https://pubmed.ncbi.nlm.nih.gov/22820313/
- Robinson, C. G. et al. Longer term results from a phase I/II study of EP-guided Noninvasive Cardiac Radioablation for Treatment of Ventricular Tachycardia (ENCORE-VT). Int. J. Radiat. Oncol. 105 682 (2019). No Title.
- Lee J, Bates M, Shepherd E, Riley S, Henshaw M, Metherall P, et al. Cardiac stereotactic ablative radiotherapy for control of refractory ventricular tachycardia: initial UK multicentre experience. Open Hear [Internet]. 2021 Nov 1 [cited 2021 Dec 11];8(2):e001770. Available from: https://openheart.bmj.com/content/8/2/e001770
- Lu J, Wang HZ, Jia Z, Zuckerman J, Lu Z, Guo Y, et al. Improving cardiac conduction with a skeletal muscle sodium channel by gene and cell therapy. J Cardiovasc Pharmacol. 2012 Jul;60(1):88–99.
- Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation [Internet]. 2012 Jan 17 [cited 2021 Dec 12];125(2):216–25. Available from: https://www.ahajournals.org/doi/abs/10.1161/circul ationaha.111.053272
- Genome UK: the future of healthcare - GOV.UK [Internet]. [cited 2021 Dec 12]. Available from:
https://www.gov.uk/government/publications/geno me-uk-the-future-of-healthcare - Cheung JW, Yeo I, Ip JE, Thomas G, Liu CF, Markowitz SM, et al. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct-Associated Ventricular Tachycardia in the Real World. Circ Arrhythm Electrophysiol [Internet]. 2018 Nov 1 [cited 2021 Dec 11];11(11):e006754. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRC EP.118.006754
Community Events Calendar