Iron Therapy in Heart Failure – The Evidence So Far
| Take Home Messages |
|---|
|
Introduction
Iron deficiency is extremely common with more than 2 billion people affected worldwide1. Its association with anaemia is well established, but its links to other diseases such as heart failure (HF) are not fully appreciated. There is now evidence of its association with HF and there have been attempts to demonstrate improvement of HF symptoms and prognosis with the replacement of iron. In this article, the physiology of iron handling and its role in HF are explored as well as the evidence for its use.
Background
Iron is a micronutrient that is crucial for a wide range of physiological processes including oxygen transport and storage, cardiac and skeletal muscle metabolism, protein synthesis and degradation, and haematopoiesis. It usually exists in two forms within the human body: the ferrous (Fe2+) form within cells and the ferric (Fe3+) form outside of cells.
Dietary iron also comes in two forms: organic haem and inorganic non-haem. Inorganic iron is absorbed by duodenal enterocytes using the divalent metal transporter 1 (DMT1) (Figure 1). There are also reductases present which reduce the ferric to ferrous form. On the other hand, haem iron is absorbed using a haem carrier protein and is reduced by haemoxigenase 1. Once within the enterocyte, ferroportin transports iron into the circulation alongside hephaestin, which oxidises the ferrous iron into ferric form. Circulating ferric iron is transferrin-bound; transferrin serves as a reservoir of soluble iron and also delivers iron to target cells where it is then absorbed using transferrin receptor type 1 (TfR1). Ferritin shells are used to store iron in the liver, spleen and bone marrow2.
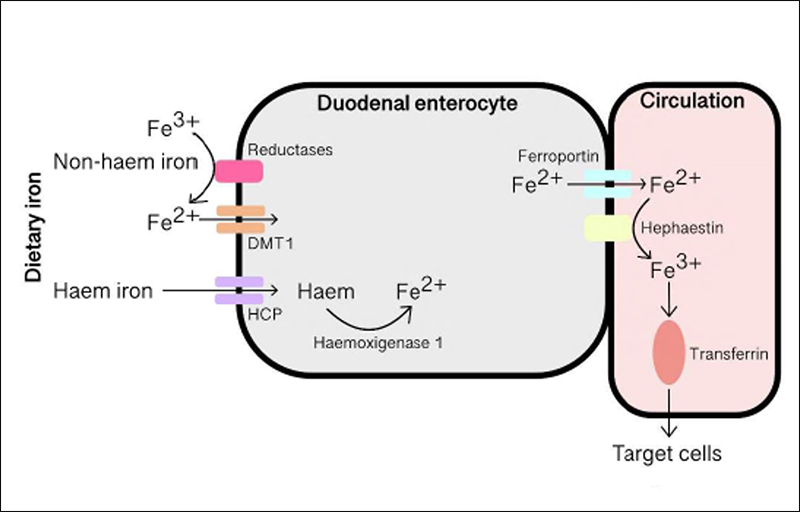
Figure 1. Absorption and metabolism of iron at the duodenal enterocyte. Fe2+, ferrous; Fe3+, ferric; DMT1,
divalent metal transporter 1; HCP, haem carrier protein.
Two types of iron deficiencies may occur: absolute and functional (see Table 1 for some causes)3. Absolute iron deficiency occurs when there is depletion of iron stores as a result of either reduced intake or loss of iron. Functional iron deficiency results from demand-supply mismatch without a low total body iron, and is thought to be due to iron being unavailable for cellular metabolism as it is trapped within the reticuloendothelial system. This usually occurs as a result of an inflammatory state.
Serum ferritin is directly proportional to iron stores in healthy individuals and therefore is used to diagnose absolute iron deficiency, with a usual cut off of <30 μg/L1. It is, however, important to understand that ferritin is also an acute phase protein and will therefore increase in acute and chronic inflammation including in patients with HF. As a result, in chronic diseases ferritin <100 μg/L is used to diagnose absolute iron deficiency. Transferrin saturation describes the percentage of transferrin that is iron-bound, and since it is a surrogate marker for the amount of iron available for use by metabolising cells, it is used for the diagnosis of functional iron deficiency, with a cut off of <20% in the presence of a normal ferritin level (100-300 μg/L).
Other markers of iron status include: reticulocyte haemoglobin, hypochromic erythrocytes, mean corpuscular volume, mean corpuscular haemoglobin, and blood haemoglobin.
| Table 1. Common causes of absolute and functional iron deficiency | |
|---|---|
| Absolute iron deficiency | Functional iron deficiency |
|
|
Iron deficiency in HF and its impact
Iron deficiency, both absolute and functional, is prevalent in patients with HF, with absolute iron deficiency being more common4. Albrecht et al5 reported iron deficiency in 26% of 100 chronic HF patients with mean left ventricular ejection fraction (LVEF) of 33.1 ± 9.7%. Jankowska and colleagues6 reported a prevalence of 37% in 546 patients with chronic HF (LVEF 26 ± 7%). Interestingly, the mean age for both cohorts was relatively low (61 and 55 respectively) and an older group may be expected to have a higher prevalence of iron deficiency. Iron deficiency has also been demonstrated in patients with HF with preserved ejection fraction (HFpEF) in similar proportions to patients to HF with reduced ejection fraction (HFrEF)7 – a systematic review and meta-analysis in 20198 showed a prevalence of 59% in HFpEF (n=1424).
The causes of iron deficiency in HF relate to several mechanisms. Firstly, HF patients have been shown to have reduced dietary intake of iron with Hughes et al. showing a reduced intake compared to reference values, particularly in patients with more advanced HF symptoms9. Secondly, rat models of dietary iron deficiency without HF are able to counteract the deficiency with upregulation of elements of the duodenal absorption pathway, such as DMT1 and ferroportin, whereas rat models with HF could not10. An analogous failure to upregulate gut absorptive mechanisms may contribute to iron deficiency in HF patients, although this is yet to be confirmed directly in humans.
Iron deficiency has been shown to be a predictor of prognosis in HF6. In Jankowska et al’s cohort, 3- year survival was 59% in patients with iron deficiency compared to 71% in patients without, and was found to be a strong independent risk factor for all-cause mortality in multivariable analyses accounting for confounders (age, sex, body mass index, NT-proBNP, NYHA class, LV EF, Creactive protein, renal function, diabetes mellitus, and presence of anaemia). Okonko et al4 looked at 157 patients with chronic HF (LVEF 32 ± 9%) and reported increased mortality over a median follow up of 2 years (hazard ratio 3.38, CI 1.48-7.72) in patients with iron deficiency compared to patients without.
Iron deficiency also impacts on symptoms of HF such as exercise capacity. The presence of iron deficiency in HF has been associated with reduced peak oxygen consumption in patients with anaemia and without anaemia11 and a few interventional trials have now demonstrated improvement of exercise capacity with intravenous iron therapy12,13.
Evidence for treatment
The evidence for correcting iron deficiency in patients with HF mainly comes from three randomised, double-blind, placebo-controlled trials: FAIR-HF, CONFIRM-HF, and AFFIRM-AHF (see Table 2).
In FAIR-HF12, 459 patients with chronic HF, New York Heart Association (NYHA) class II or III, LVEF ≤40% and iron deficiency (as defined by ferritin <100 μg/L or 100-299 μg/L with a transferrin saturation <20%), were randomised to intravenous ferric carboxymaltose (FCM) or placebo (2:1 ratio). The primary end-point was a self-reported Patient Global Assessment (PGA) and NYHA functional class at 24 weeks. The mean age for the treated group (n=304) was 67.8 ± 10.3 compared to 67.4 ± 11.1 in the placebo group (n=155) and mean LVEF was 31.9 ± 5.5 vs. 33.0 ± 6.1%.
At the end of the study period, significantly more patients in the treated group had improved PGA scores compared to the placebo group (50% vs 28%, OR 2.51, CI 1.75-3.61). There were also significant improvements in NYHA class (47% vs 30%, OR 2.40, CI 1.55-3.71) and 6-minute walk test (6MWT) distance compared to placebo.
This trial demonstrated some promising results with improvements in subjective outcomes, although there were no significant differences found in hard outcomes such as death or hospitalisations. Of note, the follow-up period was short (24 weeks), which may not have been long enough to demonstrate significant differences in death or hospitalisation. Although the study was not powered to look for such differences, there was a trend towards reduced rate of first hospitalisation due to cardiac cause. This study had a small number of participants compared with the sorts of numbers we are used to in major, opinion-changing HF trials, for example 8442 in PARADIGM-HF14 and 4744 in DAPAHF15. Patients in FAIR-HF were mainly NYHA class III (82.6% of the treatment arm) suggesting sicker patients, but the investigators did not record natriuretic peptides which may have corroborated this. A pre-specified sub-group analysis showed benefit of iron therapy regardless of the presence of anaemia, which was defined as a haemoglobin ≤120g/L at baseline16.
| Table 2. Recent randomised, placebo-controlled trials of iron therapy in HF | ||||||
|---|---|---|---|---|---|---|
| Trial | Cohort | Participants | Treatment | Primary Outcome | Follow Up | Result |
| FAIR-HF | Chronic HF, LVEF ≤40% | 459 | IV FCM 200mg weekly, then 4-weekly maintenance | PGA & NYHA class | 24 weeks | Improved PGA scores and NYHA class |
| CONFIRM-HF | Chronic HF, LVEF ≤45% | 304 | IV FCM, dose based on weight and Hb | 6MWT distance | 52 weeks | Improved 6MWT distance |
| AFFIRM-HF | Acute HF, LVEF <50% | 1110 | IV FCM, dose based on weight and Hb | HF hospitalisations & CV death composite | 52 weeks | No significant difference |
| IV, intravenous; FCM, ferric carboxymaltose; IIM, iron isomaltoside; HF, heart failure; LVEF, left ventricular ejection fraction; CV, cardiovascular; 6MWT, 6-minute walk test; PGA, patient global assessment; NYHA, New York Heart Association; Hb, haemoglobin | ||||||
CONFIRM-HF13 was a randomised, double-blind placebo controlled trial looking at the use of intravenous FCM in patients with HF. The trial randomised 304 patients with known HF with an LVEF ≤45%, raised natriuretic peptides and with iron deficiency (ferritin <100 μg/L or ferritin 100-300 μg/L with transferrin sats <20%), to have either FCM or placebo (1:1 ratio) for 52 weeks. The primary endpoint was change in 6MWT distance at week 24 compared to baseline. They also assessed NYHA class status, PGA, Fatigue Score and importantly, rates of hospitalisation as secondary outcomes. At baseline, the treatment arm had a mean LVEF of 37.1% compared to 36.5% in the placebo arm.
FCM resulted in a significant improvement in primary outcome (change in 6MWT distance +18 ± 8 m vs. -16 ± 8 m, p=0.002). Secondary endpoints also showed promising results with improvements in PGA from week 12 onwards and NYHA class from week 24. Interestingly, there was also a significant drop in the number of hospitalisations due to worsening HF (10 vs 32, OR 0.39, p=0.009), although disappointingly no difference in mortality.
This study adds to the findings of FAIR-HF and answers some outstanding questions. In CONFIRMHF there was an objective primary endpoint rather than subjective measures and this primary endpoint was met convincingly. The follow-up period of 1 year was longer than in FAIR-HF providing evidence of longer term use and benefit. In FAIRHF, the dosing was of 200 mg intravenous FCM every week until iron levels were replete, followed by 4-weekly maintenance therapy. This dosing regime is fairly complex and difficult to provide; in comparison, CONFIRM-HF used a simpler regime based on weight and Hb level17, which resulted in 75% of patients needing 2 or less injections during the study period. Above all, we also saw a significant reduction in hospitalisation from HF in this study, which may be the crucial missing piece in the puzzle that will persuade clinicians to advocate its use in HF.
CONFIRM-HF also had a greater proportion of patients with NYHA class II symptoms (53% in treatment arm) compared to the FAIR-HF study suggesting that the results also apply to less sick patients. Levels of natriuretic peptides, however, were higher than in PARADIGM-HF (NT-proBNP 2511 pg/mL vs 1631 pg/mL).
More recently, AFFIRM-AHF18 looked at patients who were stabilised after an acute HF episode with an LVEF <50% and iron deficiency (ferritin <100 μg/L or ferritin 100-299 μg/L with transferrin saturation <20%). In this randomised, double-blind, placebo controlled trial, the primary outcome was a composite of total HF hospitalisations and cardiovascular (CV) death up to 52 weeks after randomisation. A total of 1110 patients commenced treatment with intravenous FCM or placebo (1:1). The mean LVEF in the treated group was 32.6% compared to 32.7% in the placebo group, and most patients were NYHA class II or III. As expected given their recent acute decompensation, these patients had higher natriuretic peptide levels (mean NT-proBNP 4743 pg/mL in the treated arm and 4684 pg/mL in the placebo arm).
The trial failed just short of meeting its primary endpoint with a relative risk of total HF hospitalisations and CV deaths of 0.79 with treatment compared to placebo (p=0.059). The analysis of secondary endpoints, therefore, should be taken with caution but did demonstrate significant improvement in total HF hospitalisations (31.72 per 100 patient years vs. 43.15, p=0.013). Overall, these studies have provided evidence for the use of iron infusion therapy in patients with HF with systolic impairment, at least in terms of reduced symptoms and hospitalisations from HF. It is still unclear whether iron therapy reduces mortality in HF but future trials will hopefully address that important question.
Guidelines
The European Society of Cardiology (ESC) guidelines in 201619 took FAIR-HF and CONFIRM-HF into account and listed intravenous FCM as a class IIa recommendation in patients with iron deficiency and symptomatic HFrEF. The American guidelines produced a focused update20 stating the use of intravenous iron therapy in these patients as a class IIb recommendation. Of note, there are some considerations before the use of intravenous iron therapy is prescribed. There are some common side effects which include headache, dizziness, flushing, hypertension, hypophosphataemia and injection site reactions. There is a risk of hypersensitivity reactions although the risk of an anaphylactic reaction with IV FCM is thought to be rare (between 0.1-0.01%) but resuscitation facilities should be available at time of administration. It is advised to avoid its use during ongoing bacteraemia and use it with caution in patients with acute infections21.
Oral iron
Lewis et al. tested oral iron replacement in 225 patients with HFrEF (LV EF <40%) and iron deficiency (ferritin 15-100 μg/L or ferritin 101-299 μg/L with transferrin saturation <20%) in a randomised, double-blind, placebo controlled trial22 and found no significant difference in exercise capacity at 16 weeks, as measured by peak VO2, 6MWT, Kansas City Cardiomyopathy Questionnaire score or NT-proBNP levels. The limitations of oral iron therapy, including gastrointestinal side effects leading to limited tolerability, in addition to the length of time oral iron takes to replenish iron stores, mean it is unlikely to have a use in HF23.
Future work
There are several clinical trials underway (Table 3), mostly looking at the use of iron therapy in patients with chronic HF with systolic impairment using hard outcomes such as CV death or HF hospitalisation. Of interest, IRONMAN will use a different type of iron therapy, intravenous iron isomaltoside (IIM), compared to FCM used in the above mentioned trials. There has been little evidence supporting the use of iron therapy in patients with HFpEF and it will be interesting to see if FAIR-HFpEF is able to provide positive data. Although this is a smaller trial, it will hopefully provide the stepping stone to a larger trial looking at hard outcomes in a group of patients that currently lacks prognostic therapy.
| Table 3. Ongoing clinical trials for the evaluation of iron therapy in HF | |||||
|---|---|---|---|---|---|
| Trial | Cohort | Participants | Treatment | Primary Outcome | Follow Up |
| FAIR-HF2 | Chronic HF, LVEF ≤45% | 1200 | IV FCM | HF hospitalisation, CV death | 1 year |
| HEART-FID | Chronic, LVEF ≤40% | 3014 | IV FCM | Death, HF hospitalisation, 6MWT | 1 year |
| IRONMAN | Chronic, LVEF ≤45% | 1300 | IV IIM | CV death, HF hospitalisation | 2.5 years |
| FAIR-HFpEF | Chronic, LVEF ≥45% | 200 | IV FCM | 6MWT | 1 year |
| IV, intravenous; FCM, ferric carboxymaltose; IIM, iron isomaltoside; HF, heart failure; LVEF, left ventricular ejection fraction; CV, cardiovascular; 6MWT, 6-minute walk test | |||||
Conclusions
Iron deficiency is clearly prevalent in HF. There has been significant progress in the evidence for the use of intravenous iron therapy in patients with HF with systolic dysfunction, so much so that it is now part of international guidelines. There remains to be seen any evidence to show that it has mortality benefit, although there certainly appears to be enough evidence for symptomatic improvement for patients. Hard outcomes i.e. HF hospitalisations and CV death will be assessed in the ongoing trials.
Furthermore, we do not know the impact of iron infusion therapy on specific causes of HF. Most of the trials excluded patients with uncorrected significant valvular heart disease, recent acute coronary syndrome or cardiac surgery and therefore we don’t know the effect in these groups of patients. We also don’t know whether it will help patients with HFpEF.
Since the conclusion of the some of the iron therapy trials, the HF world has been shown significant evidence for the newer anti-diabetic medications such as the SGLT2 inhibitors. We therefore don’t know whether the impact of iron therapy in the current generation of HF patients, who are now on current “optimal” medical therapy, will be the same as was the case a few years ago.
Disclosures
None declared
References
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–520.
- Jankowska EA, Von Haehling S, Anker SD, MacDougall IC, Ponikowski P. Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectives. Eur. Heart J. 2013;34:816–826.
- Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut 2011;60:1309–1316.
- Okonko DO, Mandal AKJ, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: Prevalence, predictors, and relation to anemia, exercise capacity, and survival. J. Am. Coll. Cardiol. 2011;58:1241–
1251. - Adlbrecht C, Kommata S, Hülsmann M, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur. Heart J. 2008;29:2343–2350.
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010;31:1872–1880.
- Witte KKA, Desilva R, Chattopadhyay S, Ghosh J, Cleland JGF, Clark AL. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am. Heart J. 2004;147:924–930.
- Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Open Hear. 2019;6:1–9.
- Hughes CM, Woodside J V., McGartland C, Roberts MJ, Nicholls DP, McKeown PP. Nutritional intake and oxidative stress in chronic heart failure. Nutr. Metab. Cardiovasc. Dis. 2012;22:376–382. Available at: http://dx.doi.org/10.1016/j.numecd.2010.08.006.
- Naito Y, Tsujino T, Fujimori Y, et al. Impaired expression of duodenal iron transporters in Dahl salt-sensitive heart failure rats. J. Hypertens. 2011;29:741–748.
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J. Card. Fail. 2011;17:899–906.
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009;361:2436–2448.
- Ponikowski P, Van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015;36:657–668.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014;371:993–1004.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381:1995–2008.
- Filippatos G, Farmakis D, Colet JC, et al. Intravenous ferric carboxymaltose in iron-Deficient chronic heart failure patients with and without anaemia: A subanalysis of the FAIR-HF trial. Eur. J. Heart Fail. 2013;15:1267–1276.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Rationale and design of the CONFIRM-HF study: a doubleblind, randomized, placebo-controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Hear. Fail. 2014;1:52–58.
- Ponikowski P, Kirwan B-A, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396:1895–1904.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. J. Am. Coll. Cardiol. 2017;70:776–803.
- Emc. Ferinject (ferric carboxymaltose). 2020. Available at: https://www.medicines.org.uk/emc/product/5910/. Accessed April 20, 2021.
- Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency the IRONOUT HF randomized clinical trial. JAMA - J. Am. Med. Assoc. 2017;317:1958–1966.
- Mordi IR, Tee A, Lang CC. Iron Therapy in Heart Failure: Ready for Primetime? Card. Fail. Rev. 2018;4:1.
Community Events Calendar


