European Society of Cardiology Heart Failure Guidelines 2021: What should we be doing in current practice?
| Take Home Messages |
|---|
The recommendations for clinicians from ESC HF Guidelines 2021 most likely to impact management of the heart failure patient:
|
Introduction
The 2012 European Society of Cardiology (ESC) Heart Failure (HF) guidelines recommended, in its 61 pages, the use of three different drug classes for the treatment of “systolic” HF while acknowledging “diastolic” HF as a separate and untreatable entity1. The 2021 ESC-HF guidelines, in its 128 pages, cover a period of scientific study during which the understanding and treatment of HF has advanced more than for any other chronic condition2.
HF is increasingly treated by a wide range of clinicians and this editorial attempts to distil the most recent guideline into 5 key “take-home” messages.
“All at once” heart failure treatment
The 2021 guideline marks a significant change in HF management. It advises that once diagnosed, patients with HF and a reduced ejection fraction (HeFREF) should be treated with two renin-
angiotensin-aldosterone-system inhibitors (either an angiotensin converting enzyme inhibitor (ACEI), an angiotensin receptor blocker (ARB), or sacubitril-valsartan; and a mineralocorticoid receptor antagonist (MRA)); a beta-blocker; and a sodium-glucose co-transporter-2 inhibitor (SGLT2I); plus diuretic for those with venous congestion (Table 1).
| Table 1. “Quadruple Therapy” for HeFREF (adapted from McDonagh et al, 2021)2 | ||||
|---|---|---|---|---|
| Treatment | Drug Class | Drug | Starting Dose | Target Dose |
| 1 | ACEI | Enalapril | 2.5mg BD | 10-20mg BDa |
| Ramipril | 2.5mg BD | 5mg BDb | ||
| ARB | Candesartan | 4mg OD | 32mg OD | |
| Losartan | 50mg OD | 150mg OD | ||
| ARNI | Sacubitril-Valsartan | 49/51mg BDc | 97/103mg BD | |
| 2 | β-Blocker | Carvedilol | 3.125mg BD | 25mg BDd |
| Bisoprolol | 1.25mg OD | 10mg OD | ||
| 3 | MRA | Spironolactone | 25mg ODe | 50mg OD |
| Eplerenone | 25mg OD | 50mg OD | ||
| 4 | SGLT2I | Dapagliflozin | 10mg OD | |
| Empagliflozin | 10mg OD | |||
a Although the ESC list other ACEI as being licenced for the treatment of HeFREF, only trials of enalapril are listed in the supplementary appendix of the 2021 guidelines b Commonly used but with little evidence to support its use over enalapril in patients with HeFREF c Starting doses of 24/26mg BD permissible in “selected patients” – in practice, those most likely to experience side effects such as symptomatic hypotension d Target dose should be 50mg BD in patients weighing >85mg e Optional starting dose of 12.5mg OD in patients in whom there are concerns regarding renal function or hyperkalaemia. Abbreviations used: ACEI – angiotensin receptor blocker; ARB – angiotensin receptor antagonist; ARNI – angiotensin receptor neprilysin inhibitor; MRA – mineralocorticoid receptor antagonist; SGLT2I – sodium glucose co-transporter-2 inhibitor; mg – milligram; OD – once daily; BD – bis in die (twice daily) | ||||
Treatment with all four drug classes is associated with an extra 3 years of life free from HF hospitalisation or death for an 80 year old, and an extra 8 years for a 55 year old with HeFREF compared to treatment with an ACEI or ARB and beta-blocker alone (3). Each treatment has additive prognostic benefit independent of the others3,3 and the guidelines thus recommend abandoning the notion that drugs such as MRA or ARNI should only be introduced in patients with ongoing symptoms despite treatment with an ACEI or ARB, plus beta-blocker4.
While the improvements in prognosis are remarkable and very welcome, it presents several questions such as; in what order and over what time scale should each drug be introduced? Different approaches might include low dose beta-blocker plus SGLT2I followed by sacubitril-valsartan and MRA; an alternative might be low dose sacubitril-vasartan and SGLT2I followed by beta-blocker and MRA (figure 1)5-7.
While the exact order is likely to be unimportant – and is not discussed in the guideline – those caring for the patient must not lose sight of the ultimate goal of achieving quadruple therapy, despite apparent clinical stability.
Another relevant question is who should take the lead for medicines optimisation? Heart failure specialist nurses are trained for just such a role but their availability is patchy nationally8. Primary care specialists have enormously high workloads, and specialist hospital clinics lack capacity9. Additionally, the increasing use of remote consultations, which make assessment of clinical status, blood pressure, heart rate and rhythm, and renal function difficult, present another barrier to adequate medicines optimisation.
An additional problem is that a patient recently diagnosed with HeFREF who is tolerant of all four medications is likely to be taking 5-6 tablets per day, not including diuretics or treatments for co-morbidities. Polypharmacy and its consequences, such as non-adherence10, will be unavoidable for patients with HeFREF.
Heart Failure Phenotype Definitions
The 2016 ESC-HF guidelines introduced the new concept of HF with a mid-range ejection fraction to describe those lying in the grey zone between an obviously normal left ventricular ejection fraction (LVEF) on echocardiography, and an obviously reduced LVEF.
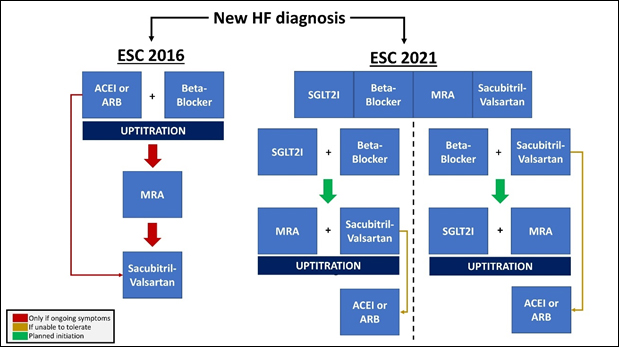
Figure 1. Comparison of ESC-HF 2016 and 2021 recommendations for medical treatment of HeFREF (adapted from Packer and McMurray, 2020; and Straw et al, 2021)6,7. ESC – European Society of Cardiology, HF – Heart Failure, HeFREF – Heart Failure and a Reduced Ejection Fraction
The 2021 iteration renames this “HF with a mildly reduced ejection fraction (HeMREF)” and gives weak recommendations for medical therapies for patient with an LVEF between 40-50% (Figure 2).
While based on some clinical data11-17, this is also an attempt to mitigate two concerns with basing treatment decisions on LVEF measured by transthoracic echocardiography:
- dividing a continuous variable (LVEF) into groups based on arbitrary cut-offs will lead to misclassification, which may mean some patients do not receive treatments that may benefit them
- there is much inter- and intra-observer variability when measuring LVEF: a patient who has an LVEF of 42% measured one day by one operator, may have an LVEF of 38% another day18.
- no trial has actually deliberately set out to investigate patients in this specific subset of patients with heart failure: all studies in patients with heart failure and normal ejection fraction have shown that the benefit of intervention increases with decreasing initial left ventricular ejection fraction
Although not in the 2021 guideline, the recent EMPEROR-Preserved study which showed a
reduction in HF hospitalisations (but not total hospitalisations) amongst patients with an LVEF <50% will almost certainly add SGLT2I to the list of treatments for patients with HeFMREF (Figure 2)14.
The term to describe patients with the HF syndrome and an LVEF >50% is controversial and, after being put to a vote, the guideline committee adopted the term “HF with a preserved ejection fration” rather than “HF with a normal ejection fraction”, despite being inaccurate. For example: a patient with an LVEF of 70% has a heart attack and later presents with breathlessness and is diagnosed with HF, an echocardiogram finds an LVEF of 55% - the LVEF cannot be described as “preserved”, but it would lie within the normal range.
Changes to Device Recommendations
Primary prevention ICD for patients with non-ischaemic cardiomyopathy
None of the landmark studies that established the prognostic benefit of implantable cardioverter defibrillator (ICD) implantation in patients with HeFREF specifically enrolled patients with non-ischaemic cardiomyopathy19-22. Those that did were either neutral23, or were closed early24,25.
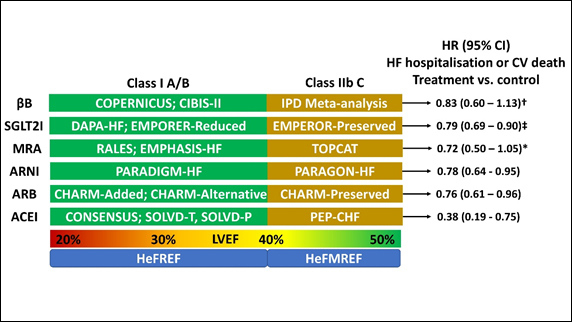
Figure 2. Medical management of HeFREF and HeFMREF (adapted from McDonagh et al, 2021)2. † - Although the combined endpoint was not statistically significant, there was a significant reduction in CV mortality with βB in patients with HeFMREF vs. placebo; ‡ - The EMPEROR-Preserved data does not feature in the 2021 ESC-HF guideline and is likely to carry a greater recommendation than level C in the next iteration; * - there was a significant reduction in the primary outcome of HF hospitalisation, aborted cardiac arrest, or CV mortality with spironolactone vs. placebo for patients enrolled in the Americas. HeFREF – heart failure and a reduced ejection fraction; HeFMREF – heart failure and a mildly reduced ejection fraction; βB – beta-blocker; SGLT2I – sodium-glucose co-transporter-2 inhibitor; MRA – mineralocorticoid receptor antagonist; ARNI – angiotensin receptor neprilysin inhibitor; ARB – angiotensin receptor blocker; ACEI – angiotensin converting enzyme inhibitor; HR – hazard ratio; CI – confidence interval; HF – heart failure; CV – cardiovascular; IPD – individual patient data.
The DANISH trial, which recruited only patients with non-ischaemic cardiomyopathy, found no reduction in all-cause mortality with ICD over usual care (although there was a signal that ICD implantation may reduce the risk of all-cause mortality in those aged 68 or younger (16% in the ICD arm vs. 21% in the control arm; HR = 0.64 (95% CI 0.45-0.90; P=0.01)26. Only 70 out of 1116 patients recruited had sudden cardiac death (SCD) over a 5 year follow up period26. Although patients with an ICD were 50% less likely to die suddenly, more patients in the ICD group had inappropriate shocks (N=34; 6%) than had SCD (N=24; 4%)26. Thus, the level of recommendation for primary prevention ICD in patients with HeFREF has been downgraded.
It is worth noting that the trials of primary prevention ICD are nearly twenty years old. In that time the rate of SCD in patients with HeFREF has fallen by 44%27, with reductions in SCD seen with ACEI, beta-blocker, MRA27, ARNI28, and SGLT2I29. ICD implantation has a complication rate between 3-9%3; although it remains a major part of HF treatment, the real prognostic benefit of primary prevention ICDs in the age of quadruple therapy is unknown.
CRT for patients with LBBB and QRS duration 130-149ms
Two individual patient-data meta-analyses from the landmark trials of CRT have suggested the prognostic benefit of CRT was less certain with a QRS duration 130-149ms, with a signal towards potential harm in patients with a QRS <130ms (Table 2)31, 32.
Although the strength of the recommendation has changed between 2016 and 2021, the data on which they are based in the referenced meta-analyses remain the same. This perhaps reflects the dilemma facing all guideline committees: should recommendations be based on the effect seen in sub-groups within a trial; or should they be based on the overall observed effect of treatment? In the case of the former, sub-group analysis may be statistically underpowered. In the case of the latter, some patients who might benefit from the treatment are excluded.
| Table 2. Estimated HR for mortality and mortality or HF hospitalisation based on QRS duration derived from an individual patient data meta-analysis in the landmark CRT clinical trials (Cleland et al, 2013)31 | ||
|---|---|---|
| QRS duration (ms) | All cause mortality HR (95% bootstrap CI) | All cause mortality or HF hospitalisation HR (95% bootstrap CI) |
| <120 | 1.20 (0.90 – 1.70) | 1.50 (0.90 – 1.80) |
| 120-139 | 1.00 (0.90 – 1.40) | 1.1 (0.90 – 1.5) |
| 140-159 | 0.90 (0.80 – 1.10) | 0.90 (0.80 – 1.10) |
| >160 | 0.80 (0.70 – 0.90) | 0.70 (0.60 – 0.80) |
| Abbreviations used: HR – hazard ratio; CRT – cardiac resynchronisation therapy; CI – confidence interval; HF – heart failure | ||
Downgrading the recommendation acknowledges the weaknesses in the evidence bases while accepting that there may be subgroups of patients who lie outside of the study entry criteria who may also benefit from the treatment.
Oral treatment on discharge
Only ~50% of patients with HeFREF admitted to hospital in the UK are discharged on triple therapy33. The reasons for this are unknown although it is probable that some patients are unable to tolerate multiple drugs that reduce blood pressure after a prolonged period of diuresis. However, the National HF Audit has consistently found that patients discharged from specialist wards are more likely to be prescribed an ACEI, ARB, MRA and beta-blocker than patients discharged from general medical wards (Table 3) and the absence of specialist knowledge or experience may be a factor33. Registry data also consistently suggests that under-treatment is a common problem in patients with HF and likely to be a result of a degree of clinical inertia34. The 2021 ESC-HF guidelines recommend that all patients admitted with heart failure are prescribed “evidence-based oral treatment” and have their oral medications “optimised” pre-discharge. It is unknown if this is realistic in practice, but it is a logical goal as those who have the most to gain from HF medications are those most at risk. For example, the readmission and mortality rates are 23% and 15% within 1 month of discharge33,35 and the prognostic benefit of HF medical therapy is seen within 30 days of initiation36-38. However, “medicines optimisation” in patients with HeFREF is often complicated, and takes many weeks to achieve. It may be more practical to develop and invest in systems that allow optimisation to take place over a series of out-patient visits shortly after discharge.
The National Institute for Health and Clinical Excellence HF guideline (and now also the ESC-HF guideline) recommend that patients are seen in clinic within 2 weeks of discharge following admission with HF; one purpose of this clinic visit is to initiate or up-titrate medications2,39. However, many HF services do not meet this target33, and efforts will be further complicated by the barriers to out-patient care as a result of the COVID-19 pandemic. Initiating quadruple therapy before discharge in patients with HeFREF may be the best opportunity to do so, whilst also providing a chance to assess tolerance.
Encouraging self-management
Self-management has been an important part of HF guidelines for years, yet 2021 is the first year to give self-management advice to patients a class I level A recommendation (Figure 4) alongside specific goals of patient education and how this might be achieved (Table 4).
Heart failure is a diagnosis for life and the specialist-patient relationship is long-term; empowering the patient through education and self-management advice at an early stage can help ensure it is, at least at the beginning, a happy one a.
| Table 3. Medications at discharge by place of care (adapted from The National Heart Failure Audit Summary Report 2018-19)33 | ||||
|---|---|---|---|---|
| Ward | ACEI/ARB | Beta-blocker | MRA | Triple therapy |
| Cardiology | 88% | 92% | 62% | 55% |
| General Medical | 80% | 85% | 45% | 35% |
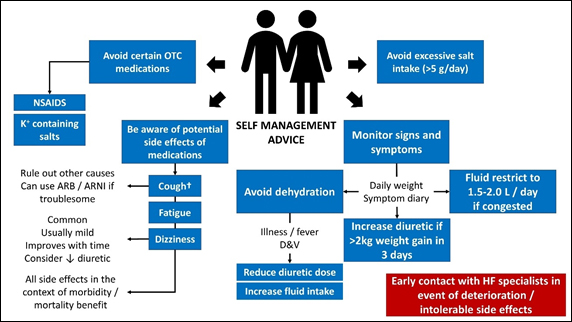
Figure 4. Self management advice for patients with heart failure (adapted from McDonagh et al, 2021)2. † - only seen with ACEI. OTC – over the counter; NSAIDS – non-steroidal anti-inflammatory drugs; ARB – angiotensin receptor blocker; ARNI – angiotensin receptor neprilysin inhibitor; D&V – diarrhoea and vomiting; g – grams; L – litre; kg – kilograms; HF – heart failure
| Table 4. Patient education goals and how to achieve them (adapted from McDonagh et al, 2021)2 | |
|---|---|
| Patient goals | How to achieve it |
| Understand the cause of heart failure |
|
| Understand the cause symptoms | |
| Understand the prognosis | |
| Understand reason for treatment |
|
| Understand common side effects | |
| Understand the importance of regular exercise |
|
Conclusions
The 2021 ESC-HF guideline has been delivered ‘with little fanfare’ but deserve recognition for its effort to incorporate recent rapid scientific advances in the field into a logical guideline for clinical practice. While the practicalities of some of the recommendations may require further discussion, for example around quadruple therapy, the rationale is persuasive. Patients with HF, and those that care for them, are being well served.
Disclosures
None
References
- McMurray JJ, Adamopoulos S, Anker SD et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803-69.
- McDonagh TA, Metra M, Adamo M et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021:ehab368. doi: 10.1093/eurheartj/ehab368. Epub ahead of print. PMID: 34447992.
- Vaduganathan M, Claggett BL, Jhund PS et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121-128.
- Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200
- Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23(6):882-894
- McMurray JJV, Packer M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation. 2021;143(9):875-877.
- Straw S, McGinlay M, Witte KK. Four pillars of heart failure: contemporary pharmacological therapy for heart failure with reduced ejection fraction. Open Heart. 2021;8(1):e001585.
- British Heart Foundation. Heart Failure Specialist Nurses. Available from: https://www.bhf.org.uk/for-professionals/healthcare-professionals/innovation-in-care/heart-failure-specialist-nurses [accessed 10/9/21]
- British Heart Foundation. Waiting Lists for Heart Patients Continue to Rise. Available from: https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2021/july/waiting-lists-for-heart-patients-continue-to-rise-nhs-figures-now {accessed 10/9/21]
- Denny RM, Hummel SL. Heart Failure Medical Management in 2020: Searching for the Right Polypharmacy. Circ Heart Fail. 2020;13(11):e007779.
- Cleland JGF, Bunting KV, Flather MD et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26-35.
- Anker SD, Butler J, Filippatos G et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021 Aug 27. doi: 10.1056/NEJMoa2107038. Epub ahead of print. PMID: 34449189.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-92
- Pfeffer MA, Claggett B, Assmann SF et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34-42
- Solomon SD, McMurray JJV, Anand IS et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381(17):1609-1620
- Yusuf S, Pfeffer MA, Swedberg K et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777-81
- Cleland JG, Tendera M, Adamus J et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-45
- Pellikka PA, She L, Holly TA et al. Variability in ejection fraction measured by echocardiography, gated single-photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Netw Open 2018;1:e181456.
- Bardy GH, Lee KL, Mark DB et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225-37.
- Moss AJ, Zareba W, Hall WJ et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-83
- Moss AJ, Hall WJ, Cannom DS et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933-40
- Buxton AE, Lee KL, Fisher JD et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882-90
23. Kadish A, Dyer A, Daubert JP et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151-8. - Bänsch D, Antz M, Boczor S et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002;105(12):1453-8.
- Strickberger SA, Hummel JD, Bartlett TG et al. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia--AMIOVIRT. J Am Coll Cardiol. 2003;41(10):1707-12
- Køber L, Thune JJ, Nielsen JC et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375(13):1221-30
- Shen L, Jhund PS, Petrie MC et al. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377(1):41-51.
- Rohde LE, Chatterjee NA, Vaduganathan M et al. Sacubitril/Valsartan and Sudden Cardiac Death According to Implantable Cardioverter-Defibrillator Use and Heart Failure Cause: A PARADIGM-HF Analysis. JACC Heart Fail. 2020;8(10):844-855.
- Curtain JP, Docherty KF, Jhund PS et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021 Aug 27:ehab560. doi: 10.1093/eurheartj/ehab560. Epub ahead of print. PMID: 34448003.
- Ezzat VA, Lee V, Ahsan S et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our 'real-world' data an underestimation? Open Heart. 2015;2(1):e000198.
- Cleland JG, Abraham WT, Linde C et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34(46):3547-56.
- Woods B, Hawkins N, Mealing S et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101(22):1800-6
- National Institute for Cardiovascular Outcomes Research. The National Heart Failure Audit Summary Report 2018-2019 (2019). Available from: https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-Report-final.pdf [Accessed 9/9/21]
- Verhestraeten C, Heggermont WA, Maris M. Clinical inertia in the treatment of heart failure: a major issue to tackle. Heart Fail Rev. 2020 May 30. doi: 10.1007/s10741-020-09979-z. Epub ahead of print. PMID: 32474794
- Martin GP, Kwok CS, Van Spall HGC et al. Readmission and processes of care across weekend and weekday hospitalisation for acute myocardial infarction, heart failure or stroke: an observational study of the National Readmission Database. BMJ Open. 2019;9(8):e029667.
- McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004
- Berg DD, Jhund PS, Docherty KF et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021;6(5):499-507.
- Lam PH, Packer M, Fonarow GC et al. Early effects of starting doses of enalapril in patients with chronic heart failure in the SOLVD Treatment Trial.Am J Med. 2020;133:e25–e31
- National Institute for Health and Clinical Excellence QS103. Chronic heart failure in adults. London: NICE, 2016. Available from https://www.nice.org.uk/guidance/qs9/chapter/List-of-quality-statements [Accessed 9/9/21]
Community Events Calendar


