The EMPEROR-Preserved Trial: The Emperor’s New Clothes?
| Take Home Messages |
|---|
|
Introduction
On the 6th July 2021, Boehringer-Ingelheim announced the preliminary positive results of the EMPEROR-Preserved trial of empagliflozin for patients with heart failure (HF) with a normal (or “preserved”) left ventricular ejection fraction (HeFNEF), declaring that EMPEROR-Preserved “…is the first and only successful trial for heart failure with preserved ejection fraction”1.
The heart failure world waited for the full publication of the study in the New England Journal of Medicine in October 2021 with great excitement2. In the event, treatment with empagliflozin was associated with a 21% reduction in the risk of first HF hospitalisation or cardiovascular (CV) death compared to placebo. Amid the hyperbole, there has been very little consideration of the detail of the EMPEROR-Preserved study and the subsequent post-hoc analyses, on which this editorial will focus.
Background
The condition
Approximately half of patients who appear superficially to have the clinical syndrome of heart failure (breathlessness, ankle swelling, fatigue) have a “preserved” or normal left ventricular ejection fraction (LVEF) on imaging3. However, establishing that a patient’s symptoms are due to cardiac dysfunction is a major challenge. There are many potential causes for “true” HeFNEF (HF symptoms due to structural heart disease with normal LVEF on echocardiogram) such as amyloidosis, hypertensive and valvular heart disease which require treatment of the underlying pathology.
There are also many potential non-cardiac diseases which may cause HF symptoms in a patient with normal-range LVEF on echocardiography, such as obesity, deconditioning, atrial fibrillation (AF), lung disease and pulmonary hypertension, each of which has to be ruled out before a diagnosis of HeFNEF can be made (Figure 1)4.
The European Society of Cardiology (ESC) Heart Failure guidelines recommend broad diagnostic criteria for HeFNEF (Figure 1) which rely predominantly on the clinical judgement that there is a high pre-test probability of HF (and the absence of other conditions) based on the presence of symptoms and signs5. The NTproBNP cut-off for excluding the diagnosis of HF in the ESC guideline is lower than the median NTproBNP of some groups of patients who have HF ruled out by thorough assessment6. Applied liberally and without thorough evaluation, the diagnosis of HeFNEF may be given to patients with diagnoses other than HF.
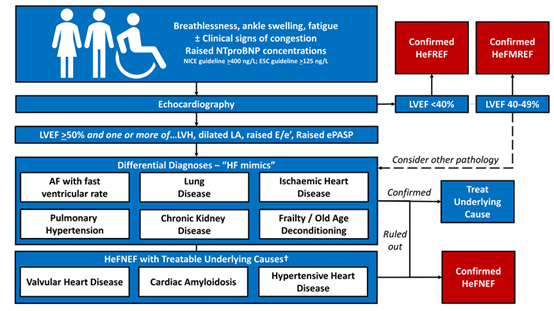
Figure 1. Establishing a diagnosis of HeFNEF. Adapted from Gevaert et al (2022)4. NTproBNP - N-terminal pro-b-type natriuretic peptide, NICE – National Institute of Health and Clinical Excellence, ESC – European Society of Cardiology, LVEF – left ventricular ejection fraction, LVH – left ventricular hypertrophy, LA – left atrium, ePASP – estimated pulmonary artery systolic pressure, AF – atrial fibrillation, HeFNEF – heart failure with a normal ejection fraction.
The treatment
Sodium glucose co-transporter 2 inhibitors (SGLT2I) were developed as anti-hyperglycaemic medications. They induce glycosuria by inhibition of the sodium glucose co-transporter in the proximal tubule (Figure 2)7.
Initially investigated in patients with diabetes, phase III studies found a reduction in HF hospitalisation regardless of the presence of HF at baseline8,9. Trials in patients with heart failure with a reduced ejection fraction (HeFREF) soon followed.
The DAPA-HF trial (N=4744, mean age 66 years, 23% female, 67% New York Heart Association (NYHA) class II, mean LVEF 31%, median N-terminal pro-B-type natriuretic peptide (NTproBNP) 1428 ng/L in the treatment arm) found that treatment with dapagliflozin was associated with a 26% reduction in HF hospitalisation or cardiovascular mortality after a median 18 months follow up. The EMPEROR-Reduced trial (N=3730, mean age 67 years, 24% female, 75% NYHA class II, mean LVEF 27%, median NTproBNP 1887 ng/L in the treatment arm) found that treatment with empagliflozin was associated with a 25% reduction in the risk of HF hospitalisation or CV mortality after a median 16 months follow up.
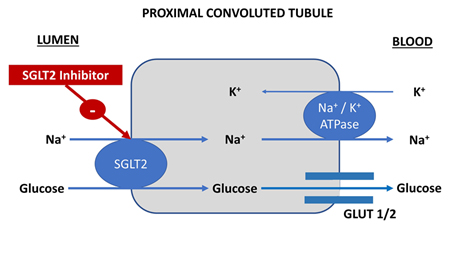
Figure 2. Mechanism of action of sodium-glucose co-transporter 2 inhibitors. SGLT2 – sodium glucose co-transporter 2.
A meta-analysis of the two trials concluded that treatment with SGLT2I in patients with HeFREF was associated with a 13% reduction in the risk of all-cause mortality, 14% reduction in the risk of CV mortality, and a 26% reduction in the risk of HF hospitalisation or CV mortality compared to placebo10.
EMPEROR-Preserved
The EMPEROR-Preserved study investigated whether the beneficial effects of SGLT2Is seen in patients with HeFREF would translate to patients with HeFNEF.
Patient population
Over 11,000 patients were screened. Of the 5595 that failed screening, the majority (78%) had an NTproBNP that was too low (box 1). Only a minority of patients failed screening for low LVEF (5%), poor renal function (2%), cardiomyopathy (1%), anaemia (1%), liver disease (1%), or AF with a heart rate >110 / min (0.3%). No patients were reported to have severe lung disease or pulmonary hypertension at screening2.
Amongst those participating in the trial (N=5988), mean age was 72 years, 45% were female, mean BMI was 30 kg/m2, approximately half of patients had AF, half had diabetes, and half had chronic kidney disease (CKD) (estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2). Mean LVEF was 54% with approximately a third of patients having an LVEF of 40-49%, a third having an LVEF of 50-59%, and a third having an LVEF of >60%. Median NTproBNP was 994 ng/L in the treatment arm2.
Findings
The primary outcome was a composite of first hospitalisation for HF or CV mortality. Secondary outcomes are shown in Box 2.
Effect on clinical outcomes
During a median follow up of 26 months, the primary outcome occurred in 13.8% of patients in the empagliflozin group and 17.1% in the placebo group: a relative risk reduction of 21% (95% confidence interval 10 – 31%) with an absolute risk reduction of 3.3%, equating to 1.8 events per 100 patient-years. The number needed to treat to prevent one CV death or hospitalisation with HF was 31 (Table 1)2.
The result was driven entirely by a reduction in HF hospitalisations: empagliflozin had no effect on mortality. Alongside a reduction in HF hospitalisation, post-hoc analyses showed a lower chance of emergency or urgent care visits for HF, or intensification of oral diuretic treatment11. The effect on HF hospitalisation reached statistical significance on day 18 after randomisation and was maintained throughout the trial10. In pre-specified sub-group analysis and post-hoc analysis of patients stratified by LVEF, the benefit of empagliflozin was seen in patients with an LVEF 40-49% and 50-59% but not in those with an LVEF >60%2,12.
Alongside a reduction in first hospitalisations for HF, there was an 8% reduction in the risk of first hospitalisations for any cause (P=0.03)11. However, there was no effect on the total number of hospitalisations for any cause (a prespecified endpoint).
| Box 1. Inclusion and Exclusion Criteria for EMPEROR-Preserved |
|---|
Inclusion
|
Exclusion
|
Derived from Anker et al (2021). Abbreviations used: NYHA - New York Heart Association, LVEF – left ventricular ejection fraction, MI – myocardial infarction, NTproBNP – N-terminal pro-B-type natriuretic peptide, LA – left atrial, LVH – left ventricular hypertrophy, HF – heart failure, BMI – body mass index, CABG – coronary artery bypass graft, LVAD – left ventricular assist device, AF – atrial fibrillation, bpm – beats per minute, ICD – implantable cardioverter defibrillator, CRT – cardiac resynchronisation device, SBP – systolic blood pressure, COPD – chronic obstructive pulmonary disease, LTOT – long term oxygen therapy, eGFR – estimated glomerular filtration rate, BCC – basal cell carcinoma. |
| Box 2. Pre-specified Primary & Secondary Outcomes |
|---|
|
| Derived from Anker et al (2021). Abbreviations used: eGFR – estimated glomerular filtration rate; KCCQ – Kansas City Cardiomyopathy Questionnaire. |
| Table 1. Notable results from EMPEROR-Preserved & post-hoc analyses | ||||
|---|---|---|---|---|
| Outcome | Empagliflozin N = 2997 | Placebo N = 2991 | Hazard Ratio (95% CI) | P |
| Primary composite endpoint | 13.8% | 17.1% | 0.79 (0.69 – 0.90) | <0.001 |
| First HF hospitalisation | 8.6% | 11.8% | 0.71 (0.60 – 0.83) | <0.001 |
| Total HF hospitalisations | 407 | 541 | 0.73 (0.61 – 0.88) | <0.001 |
| First increase in oral diuretic dose | 16.1% | 20.4% | 0.76 (0.67 - 0.86) | <0.001 |
| Total increases in oral diuretic dose | 838 | 626 | 0.73 (0.65 - 0.82 | 0.03 |
| First all-cause hospitalisation | 42.4% | 44.8% | 0.92 (0.85 – 0.99) | NS |
| Total all-cause hospitalisations | 2566 | 2769 | 0.93 (.85 – 1.01) | NS |
| Composite renal outcome | 3.6% | 3.7% | 0.95 (0.73 – 1.24) | NS |
| Incident diabetes | 12.0% | 14.0% | 0.84 (0.65 – 1.07) | NS |
| CV mortality | 7.3% | 8.2% | 0.91 (0.76 – 1.09) | NS |
| All-cause mortality | 14.1% | 14.3% | 1.00 (0.87 – 1.15) | NS |
| Data derived from references 2 and 10. N – number, CI – confidence interval, HF – heart failure; CV - cardiovascular | ||||
Empagliflozin had no effect on the composite renal outcome or incidence of diabetes. Although the rate of decline of eGFR was slower with empagliflozin compared to placebo, this was offset by an early initial drop in eGFR in the empagliflozin arm soon after randomisation: ultimately, at the end of the trial, the adjusted mean change in eGFR from baseline was -8 ml/min/1.73m2 in both groups2.
Effect on Symptoms and Quality of Life
Although more than 4 in 5 patients had only NYHA class II symptoms2, those taking empagliflozin had greater likelihood of reducing NYHA class11. Approximately half of patients in both arms experienced a >5 point improvement in the KCCQ score; a slightly greater proportion in the empagliflozin arm compared to placebo (51.6% vs. 46.5%; P<0.05)13. This is, perhaps, to view the results through rose-tinted spectacles: there was no effect on the overall mean KCCQ summary score or KCCQ score in the physical domain2,13. Consistent with this finding are the results of the EMPERIAL-Preserved trial of empagliflozin vs. placebo in patients with HeFNEF which found no difference in 6-minute walk test distance or KCCQ score after 12 weeks of treatment with empagliflozin compared to placebo14.
Interpretation
The goal of a heart failure treatment is to reduce HF-related events such as cardiovascular death, hospitalisation with HF and worsening symptoms. EMPEROR-Preserved was only partially successful in this respect. The online supplementary data show the Kaplan-Meir curves for all cause and cardiovascular mortality: both showing no effect of empagliflozin2. Heart failure as a cause of death affected less than 2% of all patients, and accounted for only around 11% of all deaths. Death from cancer was equally likely, even though patients with active malignancy were excluded. The number of non-HF hospitalisations was over 4 times greater than the number of HF hospitalisations and there was no effect on overall hospitalisations.
One of the most important findings of EMPEROR-preserved is thus that the greatest risk patients with HeFNEF face may not be HF-related events. Only half of readmissions in the 5 years after discharge for patients admitted with HeFNEF are due to heart failure15, and non-cardiovascular death is more common than cardiovascular death, and far more common than death due to heart failure16. In the last year of life of patients with HeFNEF, admissions for non-cardiovascular causes far outweigh those for cardiovascular causes17,18.
EMPEROR-preserved showed that treatment with empagliflozin reduced the risk of an uncommon event (HF hospitalisation) in patients with HeFNEF, but had no overall effect on all-cause morbidity or mortality: empagliflozin changed the reason for hospitalisation rather than reducing the overall hospitalisation rate.
The possible quality of life and symptom benefits of empagliflozin in the EMPEROR-Preserved trial are difficult to interpret: empagliflozin was associated with an increased likelihood of improving NYHA class2. Over 4 in 5 patients in the empagliflozin group had NYHA class II symptoms2, thus, in the majority, empagliflozin was associated with patients transitioning from being mildly symptomatic to being asymptomatic. However, empagliflozin had no effect on the physical limitation domain of the KCCQ13, and no overall effect on KCCQ score2. These two findings appear conflicting; any effect on quality of life is very modest.
The applicability of EMPEROR-Preserved to clinical practice in the UK is limited. Establishing an unequivocal diagnosis of HeFNEF is difficult in practice as there is a huge overlap with other conditions. There are two proposed diagnostic algorithms for HeNEF HFA-PEFF and H2FPEF19,20, but neither has consistent diagnostic accuracy when applied to populations of patients with HeFNEF21,22,23,24. Patients with HeFNEF represent a heterogenous group of patients with multiple co-morbidities such as AF, hypertension, chronic kidney disease, COPD, obesity, deconditioning, frailty and diabetes. Such complexity cannot be captured in a simple diagnostic calculator. That there is debate over the name of the condition – HF with a normal vs. persevered ejection fraction – is indicative of how poorly these complexities are understood.
NT-proBNP rises with age and comorbidities posing further problems in establishing a diagnosis of HeFNEF: NT-proBNP greater than 300 ng/L (entry criteria for patients in sinus rhythm) is extremely common in the over-80s, assuming that the age of the participants in EMPEROR-Preserved was normally distributed at least one in six would be aged over 80. For patients in AF, the NT-proBNP entry threshold was 900 ng/L however values of >900 ng/L are almost universal in patients with AF25,26.
SGLT2Is have an undoubted diuretic effect27, and trial data show that treatment of the co-morbidities associated with HeFNEF with diuretic agents reduces the risk of HF hospitalisation (Figure 3)28,29,30,31. The EMPEROR-Preserved investigators do not report the use of loop or thiazide diuretics in the treatment arms – this is an important confounding factor that needs to be clarified.
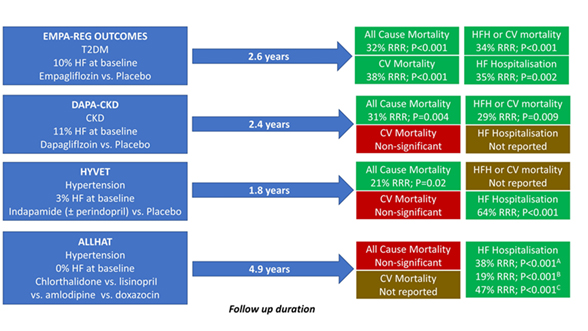
Figure 3. Clinical outcomes in trials of diuretic agents in patients with T2DM, CKD, or hypertension derived from references 28-31. A – chlorthalidone vs. amlodipine; B – chlorthalidone vs. lisinopril; C – chlorthalidone vs. doxazocin. T2DM – type 2 diabetes mellitus; HF – heart failure; RRR – relative risk reduction; CV – cardiovascular mortality; HFH – heart failure hospitalisation; CKD – chronic kidney disease; ACEI – angiotensin converting enzyme inhibitor
Conclusion
In many ways the results of the EMPEROR-Preserved trial are unsurprising - a diuretic agent reduces the risk of worsening fluid retention. However, hospitalisation for fluid retention is an uncommon event even in patients meeting the trial definition of HeFNEF. SLGT2Is are commonly used for patients with diabetes, and may soon be recommended for other co-morbidities common in patients with HeFNEF, such as CKD. Thus many patients with a label of HeFNEF may end up receiving SGLT2Is for a reason other than HF in the years to come. Regarding the use of empagliflozin specifically for the treatment of HeFNEF, amongst the clamour, the data are far from conclusive.
References
- https://www.boehringer-ingelheim.com/press-release/emperor-preserved-heart-failure-toplineresults
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451-1461.
- van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18(3):242-52
- Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, Shah SJ, Van Spall HGC. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022:heartjnl-2021-319605
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
- Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, Petutschnigg J, Ahmed FZ, Cosmi F, Brunner La Rocca HP, Mamas MA, Clark AL, Edelmann F, Pieske B, Khan J, McDonald K, Rouet P, Staessen JA, Mujaj B, González A, Diez J, Hazebroek M, Heymans S, Latini R, Grojean S, Pizard A, Girerd N, Rossignol P, Collier TJ, Zannad F; HOMAGE Trial Committees and Investigators. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart 'OMics' in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021;42(6):684-696.
- Brown E, Rajeev SP, Cuthbertson DJ, Wilding JPH. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019;21 Suppl 2:9-18..
- Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018 Jan 23;137(4):323-334. doi: 10.1161/CIRCULATIONAHA.117.032038. Epub 2017 Nov 13. PMID: 29133604; PMCID: PMC5777572.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117-28.
- Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819-829.
- Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144(16):1284-1294.
- Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2021 Dec 8:ehab798. doi: 10.1093/eurheartj/ehab798. Epub ahead of print. PMID: 34878502.
- Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, Ponikowski P, Shah SJ, Senni M, Vedin O, Verma S, Peil B, Pocock SJ, Zannad F, Packer M, Anker SD. Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation. 2022;145(3):184-193.
- Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva-Cardoso J, Störk S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42(6):700-710
- Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486. .
- Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017;69(5):556-569..
- Dunlay SM, Redfield MM, Jiang R, Weston SA, Roger VL. Care in the last year of life for community patients with heart failure. Circ Heart Fail. 2015;8(3):489-96.
- Abel A, Samuel N, Cuthbert JJ, Kazmi S, Cleland JGF, Clark AL. Hospital admissions in the last year of life in Patients with heart failure. Heart. 2021;107supp1:119-120. DOI:10.1136/heartjnl-2021-BCS.117
- Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297-3317
- Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138(9):861-870
- Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and Implications of the H2FPEF Score in a Cohort of Patients With Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139(15):1851-1853.
- Sepehrvand N, Alemayehu W, Dyck GJB, Dyck JRB, Anderson T, Howlett J, Paterson I, McAlister FA, Ezekowitz JA. External Validation of the H2F-PEF Model in Diagnosing Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2019;139(20):2377-2379. .
- Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens M, Heymans S, Beussink-Nelson L, Shah SJ, van Empel VPM. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):413-421.
- Sanders-van Wijk S, Barandiarán Aizpurua A, Brunner-La Rocca HP, Henkens MTHM, Weerts J, Knackstedt C, Uszko-Lencer N, Heymans S, van Empel V. The HFA-PEFF and H2 FPEF scores largely disagree in classifying patients with suspected heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(5):838-840.
- Shelton RJ, Clark AL, Goode K, Rigby AS, Cleland JG. The diagnostic utility of N-terminal pro-B-type natriuretic peptide for the detection of major structural heart disease in patients with atrial fibrillation. Eur Heart J. 2006;27(19):2353-61.
- Cleland JGF, Pfeffer MA, Clark AL, Januzzi JL, McMurray JJV, Mueller C, Pellicori P, Richards M, Teerlink JR, Zannad F, Bauersachs J. The struggle towards a Universal Definition of Heart Failure-how to proceed? Eur Heart J. 2021 Jun 21;42(24):2331-2343. doi: 10.1093/eurheartj/ehab082. PMID: 33791787.
- Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation. 2020;142(18):1713-1724.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436-1446.
- Kostis JB, Davis BR, Cutler J, Grimm RH Jr, Berge KG, Cohen JD, Lacy CR, Perry HM Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278(3):212-6.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CK; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CK; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898.
Community Events Calendar


