AF screening and thromboembolic risk: How much AF is significant?
| Take Home Messages |
|---|
|
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. In Europe, the prevalence of AF is projected to more than double and could reach 17.9 million by 2060, driven in part by demographic changes from an ageing population1. AF is associated with up to a three-fold increased risk of death and five-fold increased risk of stroke2-5. AF-related strokes account for approximately a third of all strokes and tend to be more disabling, more lethal and are more likely to recur6-8.
Oral anticoagulation, when indicated, is a highly effective stroke prevention strategy with significant reductions in both morbidity and mortality9. However, a large proportion of patients are asymptomatic or may only experience brief symptomatic paroxysms of AF, which are not captured on monitoring10. AF may, therefore, remain undetected and untreated until patients experience a complication, such as an AF-related stroke or decompensated heart failure11,12.
AF screening can lead to early detection which, together with timely initiation of oral anticoagulation, may prevent AF-related strokes and death13. However, knowing who should be screened, how and for how long is unclear14. Two studies, STROKESTOP and LOOP, published simultaneously in The Lancet used different AF screening strategies in high-risk populations and offer further insights into systematic AF screening (Table 1)15,16.
| Table 1. Summary of study design and main outcomes in STROKESTOP and LOOP | ||
|---|---|---|
| STROKESTOP | LOOP | |
| Study design | Prospective randomised | Prospective randomised |
| Randomisation | 1:1 | 1:3 |
| Intervention | Intermittent single-lead ECG twice daily for 14 days | ICM (AF ≥ 6 minutes) |
| Enrolment | 2012-2014 | 2014-2017 |
| Inclusion Criteria | Age: 75-76 | Age ≥ 65 and one additional stroke risk factor |
| Exclusion Criteria | None | History of AF, ongoing or contraindications for OAC |
| Primary Endpoint | Composite of ischaemic or haemorrhagic stroke, systemic embolism, hospitalisation for bleeding, or death from any cause | Composite of stroke or systemic embolism |
| Total number of patients (intervention/control group) | 28,768 (14,387/14,381) | 6,004 (1,501/4,503) |
| CHA2DS2-VASc score | 3.5 | 4 |
| AF detection after screening | ||
| Intervention | 1953 (14.1%) [p=0.005] | 477 (31.8%) [p<0.0001] |
| Control | 1794 (12.8%) | 550 (12.2%) |
| Oral anticoagulation | ||
| Intervention | † | 455 (29.7%) [p<0.0001] |
| Control | † | 591 (13.1%) |
| Composite primary endpoint | ||
| Intervention | 4456 (31.9%) [p=0.045] | 67 (4.5%) [p=0.11] |
| Control | 4616 (33.0%) | 251 (5.6%) |
| Ischaemic stroke | ||
| Intervention | 766 (5.5%) [p=0.084] | ‡ |
| Control | 830 (5.9%) | ‡ |
| Haemorrhagic stroke | ||
| Intervention | 137 (0.98%) [p=0.27] | 11 (0.8%) [p=0.94] |
| Control | 155 (1.1%) | 34 (0.8%) |
| Major bleeding | ||
| Intervention | 1431 (10.2%) [p=0.60] | 65 (4.3%) [p=0.11] |
| Control | 1448 (10.3) | 156 (3.5%) |
| All-cause death | ||
| Intervention | 3177 (22.7%) [p=0.12] | 168 (11.2%) [p=1.00] |
| Control | 3287 (23.5%) | 507 (11.3%) |
| AF, atrial fibrillation. ICM, implantable cardiac monitors. OAC, oral anticoagulants. † Total number of patients on oral anticoagulation is not available only number of patients stratified per year. ‡ Number of ischaemic strokes is not available. Composite primary endpoint combines ischaemic strokes with systemic embolism. | ||
AF screening
The likelihood of detecting new AF during screening depends upon screening intensity (single time-point, intermittent, continuous), screening strategy (opportunistic, systematic) and the demographics of the population being screened14 .
In general, the longer the monitoring window and intensity of screening, the higher the yield (Figure 1)15-24. The detection rate of new AF in individuals aged ≥ 65 years was 1.4% in a meta-analysis of 19 studies using single time-point assessment vs. a 34% yearly detection rate in the ASSERT-II study which used implantable cardiac monitors (ICM)17,23. Nevertheless, the cohort of patients diagnosed with AF on single time-point screening are likely to have a higher arrhythmia burden, and thus sit more closely with patients who have clinically apparent AF25. Though extended screening with continuous rhythm monitoring undoubtedly identifies more AF, this includes short-lasting asymptomatic AF episodes (i.e minutes) of unclear clinical significance and the episode duration or daily AF burden that merits oral anticoagulation is currently unknown.
AF screening strategies have previously been classified as opportunistic, usually during a healthcare visit, or systematic, targeting an entire population. Recent advancements in consumer-facing wearable devices (smartphone applications, smartwatches, bands or rings) have given rise to a third type of AF screening: patient-initiated screening26. These new technologies provide exciting new screening tools but there are significant knowledge gaps in their accuracy and use within the current healthcare system.
Opportunistic screening is recommended by many major medical societies5,27-29. Pulse palpation to assess for an irregular pulse followed by an electrocardiogram is recommended by NICE if AF is suspected, and by the European Society of Cardiology (ESC), in individuals aged ≥ 65 years5,27. However, there is less consensus with regards to systematic screening: ESC recommends systematic screening for individuals aged ≥ 75 years old, or at a high stroke risk ―albeit with a lower class of recommendation, Class IIa ― but UK National Screening Committee and the US Preventive Service do not29,30. The rationale for this is the lack of robust randomised hard outcome data.
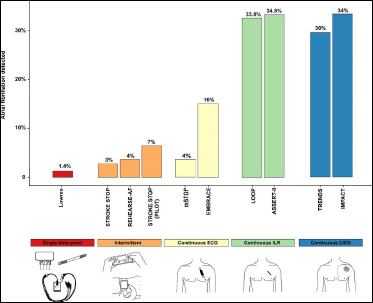
Figure 1. Rates of AF detection with different screening modalities (Adapted from Engdahl et al25)
STROKESTOP16 and LOOP15 – Headline results
The STROKESTOP study randomised 28,768 Swedish residents aged 75-76 years to intermittent AF screening or routine care (control group). Individuals assigned to screening were required to perform a 30-second ECG twice daily with a handheld device (Zenicor) for 14 days. AF was defined as an irregular rhythm without P-waves for 30 seconds or two episodes lasting 10-29 seconds each. If AF was detected or previously untreated, oral anticoagulation was offered. Notably, there was no exclusion criteria. At baseline, the control group had a slightly higher rate of AF than the screening group (12.8% vs 12.1%) but after screening, 262 (1.87%) new AF patients were identified.
Over the course of the study, screening led to a higher proportion of new AF diagnosis in the intervention arm. Although oral anticoagulation initiation in AF patients after one year was higher in the screening group (65.8% vs 59.8%, p= 0.005, respectively), this levelled out during the study. After a median follow-up 6.9 years, fewer patients in the screening group met the primary endpoint (composite of ischaemic or haemorrhagic stroke, systemic thromboembolism, severe bleeding, and all-cause mortality) than those in the control group (4456 vs 4616, respectively). The intention-treat analysis showed only a modest 4% relative risk reduction (HR 0.96: CI 0.92-1.00; p-value=0.045) in the primary endpoint which represents a number needed to screen of 91 patients. The Kaplan-Meier curves start to diverge at around four years, and the overall results appear to be driven by a reduction in ischaemic strokes in the screening arm. The authors concluded that AF screening in the elderly is safe and led to a ‘small net clinical benefit’.
The LOOP study was designed to investigate whether continuous rhythm monitoring with ICMs and oral anticoagulation for AF episodes longer than 6 minutes would prevent ischaemic strokes and systemic embolism (primary endpoint). 6,004 Danish patients aged over 70 with an additional risk factor for stroke were randomised in a one-to-three fashion to an ICM (1,501) or routine care (4,503). The median CHA2DS2-VASc was 4 and patients were followed up for a median of 16.8 months. Despite a three-fold increase in AF detection (31.8% vs 12.2%) followed by oral anticoagulation initiation (29.7% vs 13.1%) in the ICM group, there was a non-significant 20% reduction (HR 0.80; 95%CI 0.61-1.05, p = 0.11) in the primary endpoint.
Interpretation - AF burden and thromboembolic risk
How to reconcile the difference in outcomes between these two trials in a similar high-risk population? As the authors of the LOOP study concluded: ‘not all AF may be worth screening’15 ; implying that AF burden may play a significant role in the overall thromboembolic risk.
Landmark trials demonstrating the net clinical benefit of oral anticoagulants required electrocardiographic evidence of AF prior to enrolment31-34. As a result, they were more likely to include patients with persistent AF or a high burden of paroxysmal AF. A recent meta-analysis demonstrated that the adjusted and unadjusted mortality and stroke risk was higher in persistent AF, supporting the notion that AF burden is a risk modifier in clinical AF35.
Studies with cardiac implantable electronic devices (CIEDs) provide some insight into the association between AF episode duration and/or AF burden and thromboembolic risk22,36-43. The term ‘silent’ or ‘subclinical’ AF was initially coined to refer to asymptomatic AF episodes detected by CIEDs, but it is perhaps used more broadly today to include episodes captured by ICMs and wearable devices. Importantly, these devices have a varying degree of diagnostic accuracy, and all episodes require adjudication to confirm that they truly represent AF and are not false positive detections due to far-field R wave, ectopy, or other atrial tachyarrhythmias.
Patients with subclinical AF have a 5-fold increased risk of developing clinical AF and a significant yearly stroke risk (2.8/100 per person-years) albeit numerically smaller than patients with clinical AF44. The AF episode duration associated with increased thromboembolism risk varied considerably amongst studies: 5 minutes in MOST, 6 minutes in ASSERT, 1 hour in SOS, 5.5 hours in TRENDS and Turakhia et al, and 24 hours in studies by Botto et al, Cappuci et al.22,36-38,41,45
The LOOP investigators designed their study in line with ASSERT study criteria, which showed an association between subclinical AF episodes ≥ 6 minutes and thromboembolism39. However, a post-hoc analysis of the ASSERT study published in 2017 demonstrated that only episodes longer than 24 hours were associated with ischaemic strokes, and, in fact, there was no difference between patients with subclinical AF lasting 6 minutes to 24 hours and those without subclinical AF43. In the LOOP study, the AF threshold that triggered oral anticoagulation was likely too low; AF episodes ≥ 24 hours were only seen in 16% of patients which may help the non-significant reduction in ischaemic strokes observed in the study.
STROKESTOP screening strategy required participants to monitor their rhythm for 14 minutes during a 2-week period ― approximately 0.07% of the screening window. It therefore included patients with a higher AF burden in whom the stroke risk most closely resembles clinical AF. Moreover, one should take into account that the intervention in STROKESTOP was an invitation for screening and only 51.3% participated. In this ‘as-treated’ cohort, which represents a younger and healthier group, the results are more compelling with a 24% reduction in ischaemic stroke (HR 0.76; 95% CI 0.68 - 0.87; p < .001).
Other factors, in addition to AF burden, may have influenced the results of the LOOP study. The control arm had an unusually high rate of AF detected (12%); the authors had assumed a 3%detection rate in keeping with other studies, such as CRYSTAL-AF and EMBRACE-AF21,46. This may have diluted the difference in outcomes between the intervention and control arms. Compliance with the ICM was also overestimated; rates of early ICM explants were more than double than anticipated, 12% and 5%, respectively.
Two randomised controlled trials (ARTESiA, NCT01938248; NOAH AFNET 6, NCT02618577) of oral anticoagulation in subclinical AF episodes are currently ongoing and may further inform our understanding47,48.
Conclusions – What lessons are we to learn?
Taken together, these two studies strengthen our understanding of AF screening. Firstly, they demonstrate the feasibility of using new technologies with remote monitoring to screen large number of patients. Secondly, they highlight the challenges of screening invitation as an intervention, particularly amongst those in older age groups or lower-socioeconomic status ― almost half of those invited did not engage with screening in STROKESTOP. Thirdly, they provide further evidence that short-lasting AF episodes carry a lower thromboembolic risk and may not warrant oral anticoagulation and intermittent ECG monitoring may be a better strategy. Lastly, these studies reinforce current ESC guidance which upgraded systematic screening from class IIb to IIa recommendation; results of HEARTLINE, SAFER and STROKESTOP2 are eagerly awaited (Table 2).
| Table 2. Summary of selected systematic screening studies currently recruiting. | |||||||
|---|---|---|---|---|---|---|---|
| Study | Study design | Population | Sample size | Intervention | Follow-up | Primary endpoint | Funding |
| STROKESTOP 2 (NCT02743416) | RCT | 76-75 | 28,712 | Invitation for screening with handheld 30-secs ECG four times a day for 14 days combined with biomarker (NT-proBNP) | 5 years |
Incidence of stroke and systemic embolism: 1. Control vs intervention 2. Control vs low-risk (NT-proBNO <125ng/l) | Roche |
| HEARTLINE (NCT04276441) | RCT | ≥ 65 | 15,000 | Intermittent screening with iPhone or Apple Watch | 3 years |
1. Time to AF diagnosis 2. Days covered by oral anticoagulation | Jansen and Apple |
| SAFER (ISRCTN72104369) | RCT | ≥ 70 | 120,000 | Intermittent screening with handheld 30-sec ECG four times a day for 3 weeks | 5 years | Fatal or non-fatal stroke | NIHR |
| ECG, electrocardiogram. NIHR, National Institute of Health Research, PPG, photoplethysmography. RCT, randomised controlled trial | |||||||
References
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746-51.
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139-e596.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-8.
- Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45(9):2599-605.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020.
- Jorgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27(10):1765-9.
- Asberg S, Henriksson KM, Farahmand B, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke. 2010;41(7):1338-42.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community-based study. Stroke. 2005;36(11):2362-6.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-67.
- Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clinical Cardiology. 2017;40(6):413-8.
- Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482.
- Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89(1):224-7.
- Wilson JMG, Jungner G, World Health O. Principles and practice of screening for disease / J. M. G. Wilson, G. Jungner. Geneva: World Health Organization; 1968.
- Jones NR, Taylor CJ, Hobbs FDR, et al. Screening for atrial fibrillation: a call for evidence. Eur Heart J. 2020;41(10):1075-85.
- Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. The Lancet.
- Svennberg E, Friberg L, Frykman V, et al. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. The Lancet.
- Lowres N, Olivier J, Chao T-F, et al. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLOS Medicine. 2019;16(9):e1002903.
- Engdahl J, Andersson L, Mirskaya M, et al. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127(8):930-7.
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation. Circulation. 2017;136(19):1784- 94.
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. Jama. 2018;320(2):146- 55.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. New England Journal of Medicine. 2014;370(26):2467-77.
- Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474-80.
- Healey JS, Alings M, Ha A, et al. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136(14):1276-83.
- Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36(26):1660-8.
- Engdahl J, Rosenqvist M. Large-scale screening studies for atrial fibrillation - is it worth the effort? J Intern Med. 2021;289(4):474-92.
- Benjamin EJ, Go AS, Desvigne-Nickens P, et al. Research Priorities in Atrial Fibrillation Screening: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation. 2021;143(4):372-88.
- National Institute for Health and Care Excellence (2021) Atrial fibrillation: Diagnosis and Management (NICE Guideline 196). Available at https://www.nice.org.uk/guidance/ng196 [updated Last accessed 13 May 2021].
- Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace. 2017;19(10):1589-623.
- The UK NSC recommendation on Atrial Fibrilation screening in adults. https://legacyscreening.phe.org.uk/atrialfibrillation
- Curry SJ, Krist AH, Owens DK, et al. Screening for Atrial Fibrillation With Electrocardiography: US Preventive Services Task Force Recommendation Statement. Jama. 2018;320(5):478-84.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2009;361(12):1139-51.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2011;365(11):981-92.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine. 2011;365(10):883-91.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2013;369(22):2093-104.
- Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591-602.
- Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614-9.
- Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46(10):1913-20.
- Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241-8.
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical Atrial Fibrillation and the Risk of Stroke. New England Journal of Medicine. 2012;366(2):120-9.
- Shanmugam N, Boerdlein A, Proff J, et al. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012;14(2):230-7.
- Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2013;35(8):508-16.
- Witt CT, Kronborg MB, Nohr EA, et al. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015;12(12):2368-75.
- Van Gelder IC, Healey JS, Crijns H, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-44.
- Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39(16):1407-15.
- Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040-7.
- Sanna T, Diener H-C, Passman RS, et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. New England Journal of Medicine. 2014;370(26):2478-86.
- Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137-45.
- Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12-8.
Community Events Calendar


