Asymptomatic severe aortic stenosis: what do we know and where are we going?
| Take Home Messages |
|---|
|
Introduction
Valvular heart disease (VHD) is increasing in incidence1 and it is expected that the number of patients affected is due to double by 2046. Aortic stenosis (AS) is the most common valvular lesion requiring intervention2 in the United Kingdom (UK) and Europe, even in the pre-transcatheter aortic valve replacement (TAVR) era. In the UK from 2018-2019 there were >10,000 TAVR or isolated surgical aortic valve replacements (SAVR), of which TAVR has now surpassed SAVR3.
AS is often caused by progressive fibro-calcific thickening and remodelling of the aortic valve (AV) leaflets that leads to restriction and obstruction4. This subsequently induces increased afterload on the left ventricle (LV) and LV hypertrophy. This initially adaptive response eventually decompensates resulting in heart failure and death (Figure 1).
The prognosis of symptomatic severe AS is dire, with a 1-year mortality of ~50%5. At present there are no medications that ameliorate the disease progress, therefore timely valve replacement is essential for long term survival.
With regards to the management of asymptomatic AS, there is debate around the timing of AVR. Intervention should ideally take place when the risks of the disease process (irreversible myocardial damage and sudden cardiac death (SCD)) outweigh the periprocedural and long-term complications from intervention. This editorial will discuss the high-risk features of asymptomatic severe AS and future research aimed at optimising outcomes in this group.
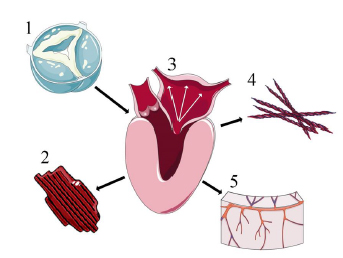 Figure 1. Myocardial impact of AS. 1. Fibrocalcific degeneration and progressive obstruction 2. Left ventricular hypertrophy 3. Diastolic dysfunction, raised left atrial pressure, and pulmonary hypertension 4. Focal and diffuse fibrosis 5. Microvascular dysfunction. Royalty free images from smart.servier.com
Figure 1. Myocardial impact of AS. 1. Fibrocalcific degeneration and progressive obstruction 2. Left ventricular hypertrophy 3. Diastolic dysfunction, raised left atrial pressure, and pulmonary hypertension 4. Focal and diffuse fibrosis 5. Microvascular dysfunction. Royalty free images from smart.servier.com
Current guidelines
The most recent iterations of major society guidelines9,10 advise intervention in AS for those symptomatic with severe haemodynamic parameters, or left ventricular systolic dysfunction attributed to the valve. A new addition acknowledges that those asymptomatic with very severe AS (Vmax >5m/s, mean gradient >60mmHg), severe valve calcification, Vmax progressing >0.3m/s/year, elevated brain natriuretic peptide (BNP)) can be considered for intervention. This is categorised as class IIa and level B evidence in both European and North American guidelines.
This approach likely remains suboptimal as there remains significant mortality (22% at 3.5 years11) after intervention, the mechanisms of which are under investigation12. Intervention may occur after irreversible cardiac damage has occurred in asymptomatic severe cases. Symptom assessment can be challenging in this population group due to the high burden of co-morbidities that confound the symptoms of AS, and physical inactivity can limit the reproduction of symptoms in activities of daily living. Here I discuss several features based on imaging, exercise testing and blood biomarkers which have been shown to indicate a high-risk subgroup of asymptomatic AS patients who may benefit from earlier intervention.
High risk features in asymptomatic severe AS
Echocardiographic haemodynamic features
Echocardiography is the cornerstone of diagnosis, surveillance, and can distinguish higher risk individuals without the requirement for additional tests. Those with very severe haemodynamic features on echocardiography in meta-analyses6,12,13 consistently report better outcomes with early intervention vs a “watchful waiting” strategy though there is a lack of randomised data and these meta-analyses rely heavily upon observational data of wide heterogeneity.
The RECOVERY trial16 randomised those with very severe AS based on echocardiographic parameters to either early surgery (predominantly SAVR) or watchful waiting. The early surgery group had no operative mortality and 1 cardiovascular death (median follow up >6 years). In comparison, 52 patients (74%) within the conservative group required intervention, of which 17% were urgent operations from unplanned admissions and cardiovascular mortality was significantly higher (n=11, 15%; p<0.01) at follow up.
The authors however noted that 1 in 4 of the conservative group did not require surgery during prolonged follow up, highlighting the heterogeneity of this group of patients.
Echocardiographic myocardial features
Myocardial deterioration in response to AS is readily detectable on echocardiography (Figure 2) and needs attention when decision making in asymptomatic patients.
AVR improves left ventricular ejection fraction (LVEF) by >10% in the majority (65-83%) even when pre-operative LVEF is <40%20. However subtle deterioration of systolic function is a marker of poor prognosis21,22 and those with asymptomatic decline to LVEF <55% can be considered for AVR9. Registry data18 comparing LVEF at presentation with conservatively managed severe AS shows significant differences in outcomes (all-cause and cardiovascular mortality, heart failure admission, SCD) comparing LVEF 50-59% against >70% (p <0.001). This effect was not seen when comparing LVEF 60-69% against >70%. In those that underwent AVR, the impact of LVEF at presentation between groups became insignificant.
Global longitudinal strain (GLS) is more sensitive at detecting myocardial dysfunction before the overt onset of impaired LVEF23. Impaired GLS is present in those with asymptomatic severe AS24 when compared to age- and sex- matched controls and deteriorates significantly (p <0.001) by -1.7%per year with no detectable reduction in LVEF. GLS >-18.2% was significantly associated with development of symptoms and requirement for AVR23.
Beyond left ventricular structure and function the myocardial impact of AS can be seen with alterations in diastology, atrial abnormalities, atrial fibrillation, pulmonary pressures, and right ventricular dysfunction25,26. Extra-valvular and cardiac damage has been classified into defined stages by Genereux et al., which correlate with a stepwise progression in mortality and hospitalisation 1 year post AVR. Those within stage 4 have a 5.5 times all-cause mortality compared to stage 0, and at the time of intervention the majority of patients with severe AS (84%) had developed at least stage 2 damage.
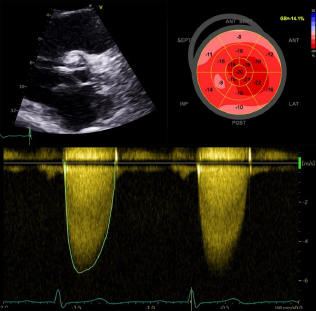
Figure 2. High risk echocardiographic features of severe AS. Top left: Severely calcified aortic valve Top right: Impaired global longitudinal strain at -14.1% Bottom: Aortic valve Vmax >5.5 m/s
Machine learning using echocardiography
Machine learning using echocardiography alone can identify high- and low-risk phenotypes that may benefit from earlier valve intervention29. A machine learning algorithm trained on an echo cohort (n=1052) correctly identified 99% of those with classical concordant severe AS as high-risk, and 64% of inconclusive patients with discordant findings into the high-risk group. When combined with cardiac magnetic resonance imaging (CMR)(n=160) high-risk phenotypes were twice as likely to have replacement fibrosis (p<0.01), high LV mass, and diffuse myocardial fibrosis. These high risk patients also had significantly more (p<0.01) aortic valve calcification (AVC) on cardiac computed tomography, were more likely to undergo AVR, and had higher mortality risk compared to the low-severity phenotype. Compared to the current standard of care, use of the algorithm resulted in improved prognostication in patients with severe AS.
Exercise testing
Exercise testing has long been used to unmask symptoms; inducible symptoms or a sustained drop of >20mmHg is considered an indication for intervention. However, the discriminatory ability of exercise testing is not particularly impressive; 21%severe AS patients with normal tests suffer adverse cardiac events, and 66% with abnormal tests go on to develop adverse events. In addition to this, 15%of patients cannot undertake exercise testing(32), nor are the potential symptoms it induces specific to AS, especially with concurrent coronary disease. Considering its limited discrimination, the results of exercise testing should be interpreted alongside results from other investigations in order to appropriately identify high-risk AS patients.
Cardiac MRI (CMR)
CMR is not included in either major society guideline, but is increasingly recognised as the modality of choice for evaluation of the myocardial impact of AS34. CMR yields accurate and reproducible structural, functional, haemodynamic assessment of AS with invaluable addition of tissue characterisation.
CMR planimetry of the aortic valve (Figure 3) corroborates (within 0.01cm2) with transoesophageal echocardiography, but tends to overestimate (by 0.17cm2) compared to TTE. Peak aortic velocity, via phase contrast imaging, correlates well with TTE (35,36) but with a tendency to under-estimate. Newer 4D-flow measurement sequences can detect higher peak and mean gradients, but long acquisition time and requiring additional software limits routine use.
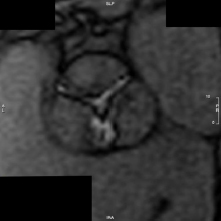
Figure 3. CMR planimetry of aortic valve. Cardiac MRI planimetry of a severely stenotic aortic valve. Multiple slices are taken through the valve to determine the minimal orifice area.
CMR can uniquely identify and quantify myocardial fibrosis, a prime driver of myocardial decompensation in AS37. There are two types of fibrosis observed in AS which can be visualised and quantified on CMR. Diffuse fibrosis, which represents cellular hypertrophy and extracellular matrix expansion33, can regress post AVR.
Focal fibrosis or scar is not reversible and is associated with 3x higher all-cause and cardiovascular mortality in patients with severe AS38,39. It has been demonstrated in 36% of asymptomatic moderate-severe AS patients40 at baseline, and 57% at 1-year follow up, despite patients remaining asymptomatic. This shows that irreversible myocardial damage, which impacts on patient prognosis, frequently occurs before the onset of symptoms.
Cardiac CT
Whereas CMR is the gold-standard to assess the myocardial response to AS; CCT can be utilised for AVC, valve and aortic morphology, valve area, and intervention planning. AVC has long been a qualitative measure of AS severity45 and can be measured via CCT using the Agaston method46. AVC reliably correlates with disease severity as measured by echocardiography, and can reliably identify severe AS using sex-specific cut-offs with a C-statistic of 0.8946. When utilised in asymptomatic AS cohorts47,48 raised AVC is an independent predictor of adverse outcome. AVC has already been integrated into the ESC guidelines where there is discordance between flow reserve and left ventricular systolic function, but perhaps can be justifiably used in asymptomatic severe AS patients to provide additional risk stratification.
Natriuretic peptides
Widespread and easily available, natriuretic peptide levels (BNP) can help identify high-risk asymptomatic AS patients. Elevated (age and sex adjusted) BNP in asymptomatic patients have a shorter symptom free survival during follow up50. Elevated BNP has been associated with a statistically significant increase in midterm (6 month to 4 year) mortality (HR1.88, 95% CI, 1.54-2.28; p <0.01) in AS patients before TAVI51. Periprocedural mortality however is unaffected.
Future Directions
There is significant evidence that the current guideline management of asymptomatic severe AS may be suboptimal for optimising long-term outcomes. There is a paucity of randomised trial data to base current guidelines in this challenging group. At present there are numerous large-scale trials designed for this population which are outlined in Table 1.
| Table 1. Ongoing trials in asymptomatic severe AS | ||||||
|---|---|---|---|---|---|---|
| Trial: | Design: | Target recruitment: | Inclusion: | Definition asymptomatic: | Primary end-point: | Expected finish: |
| EARLY TAVR52 | Prospective, randomised control trial, multicentre | 900 | Aged >65 AVA <1cm2 or AVAi <0.6cm2/m2 and AV-Vmax >4m/s or MG >40mmhg LVEF >50% STS <10 | Negative treadmill test or clinical history if unable to undertake treadmill | All-cause mortality and unplanned cardiovascular hospitalisation | 2032 |
| EVOLVED37 | Prospective, randomised control trial, multicentre | 1000 | AV-Vmax >4m/s or AVAi <0.6cm2/m2 and AV-Vmax >3.5m/s Midwall fibrosis on CMR LVEF >50% on CMR | May include exercise testing depending on attending physicians’ usual practice | Composite all-cause mortality or unplanned aortic stenosis related hospitalisation | 2024 |
| AVATAR53 | Prospective, randomised control trial, multicentre | 157 | AV-Vmax >4m/s or MG >40mmhg and AVA <1cm2 or AVAi <0.6cm2/m2 STS <8% LVEF >50% | Negative exercise test with at least 80% maximal heart rate achieved | All-cause mortality, major adverse cardiac event | 2021 |
| DANAVR54 | Prospective, randomised control trial, multicentre | 1700 | AVA <1cm2 and AV-Vmax >3.5m/s and LAVi >34ml/m2 or E/e’ avg >13 or 3x NT-proBNP (age and sex adjusted) or GLS >-15.5 | Considered to be asymptomatic by a consultant in cardiology | All-cause mortality | 2029 |
| EASY-AS55 | Prospective, randomised control trial, multicentre | 2844 | AV-Vmax >4m/s or MG >40mmHg and AVA <1cm2 or AVAi <0.6cm2/m2 LVEF >50% | Recruiting clinician discretion | Cardiovascular death and hospitalisation for heart failure | 2029 |
| AVA, aortic valve area. AVAi, indexed aortic valve area. AV-Vmax, aortic valve maximal velocity. MG, mean gradient. LVEF, left ventricular ejection fraction. STS, society of thoracic score. LAVi, left atrial volume index. NT-proBNP, brain naturetic peptide. GLS, global longitudinal strain. | ||||||
Summary
Patients presenting with asymptomatic severe AS are common, challenging, and heterogenous. The current approach of watchful waiting is supported by recent major society guidelines and there is a lack of rigorous prospective trial data in this group to warrant deviation.
There are however multiple high-risk features that the treating clinician should be wary of that can signal a patient at increased risk of symptom development or persistent long-term risk despite intervention. This includes features readily available on routine echocardiography but also additional imaging modalities and biomarkers. Numerous randomised trials ongoing, aimed at shining light on this complex issue, may cause a paradigm shift in how this patient population is managed.
References
- BHVS Blueprint [Internet]. [cited 2021 Apr 4]. Available from: https://www.bhvs.org.uk/bhvs-blueprint/
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease. European Heart Journal [Internet]. 2003 Jul;24(13):1231–43. Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1016/S0195-668X(03)00201-X
- NICOR. National Adult Cardiac Surgery Audit (NASCA) - 2020 Summary Report [Internet]. 2020. Available from: https://www.nicor.org.uk/wp-content/uploads/2020/12/National-Adult-Cardiac-Surgery-Audit-NACSA-FINAL.pdf
- Lindman BR, Clavel M-A, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nature Reviews Disease Primers [Internet]. 2016 Dec 22;2(1):16006. Available from: http://www.nature.com/articles/nrdp20166
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New England Journal of Medicine [Internet]. 2010 Oct 21;363(17):1597–607. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1008232
- R. DM, A. BN, E. ND. Calcific Aortic Stenosis. Journal of the American College of Cardiology [Internet]. 2012 Nov 6;60(19):1854–63. Available from: https://doi.org/10.1016/j.jacc.2012.02.093
- Treibel TA, Kozor R, Fontana M, Torlasco C, Reant P, Badiani S, et al. Sex Dimorphism in the Myocardial Response to Aortic Stenosis. JACC: Cardiovascular Imaging. 2018 Jul;11(7).
- Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh A, et al. Extracellular Myocardial Volume in Patients With Aortic Stenosis. Journal of the American College of Cardiology [Internet]. 2020 Jan;75(3):304–16. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109719385146
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal [Internet]. 2021 Aug 28; Available from: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab395/6358470
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation [Internet]. 2021 Feb 2;143(5). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000923
- Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, et al. Myocardial Scar and Mortality in Severe Aortic Stenosis. Circulation [Internet]. 2018 [cited 2021 Nov 13];138(18):1935–47. Available from: https://pubmed.ncbi.nlm.nih.gov/30002099/
- Mechanisms of Excess Risk in Aortic Stenosis - Full Text View - ClinicalTrials.gov [Internet]. [cited 2021 Nov 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT04627987
- Kumar A, Majmundar M, Doshi R, Kansara T, Shariff M, Shah P, et al. Meta-Analysis of Early Intervention Versus Conservative Management for Asymptomatic Severe Aortic Stenosis. The American Journal of Cardiology. 2021 Jan 1;138:85–91.
- Lim WY, Ramasamy A, Lloyd G,Bhattacharyya S. Meta-analysis of the impact of intervention versus symptom-driven management in asymptomatic severe aortic stenosis. Heart [Internet]. 2017 Feb 15 [cited 2021 Oct 23];103(4):268–72. Available from: https://heart.bmj.com/content/103/4/268
- Yokoyama Y, Takagi H, Kuno T. Early surgery versus conservative management of asymptomatic severe aortic stenosis: A meta-analysis. The Journal of Thoracic and Cardiovascular Surgery. 2020 Jul 5;
- Kang D-H, Park S-J, Lee S-A, Lee S, Kim D-H, Kim H-K, et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. https://doi.org/101056/NEJMoa1912846 [Internet]. 2019 Nov 16 [cited 2021 Oct 23];382(2):111–9. Available from: https://www.nejm.org/doi/10.1056/NEJMoa1912846
- Lancellotti P, Magne J, Dulgheru R, Clavel M-A, Donal E, Vannan MA, et al. Outcomes of Patients With Asymptomatic Aortic Stenosis Followed Up in Heart Valve Clinics. JAMA Cardiology [Internet]. 2018 Nov 1 [cited 2021 Oct 8];3(11):1060–8. Available from: https://jamanetwork.com/journals/jamacardiology/fullarticle/2703125
- Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Prognostic Impact of Left Ventricular Ejection Fraction in Patients With Severe Aortic Stenosis. JACC: Cardiovascular Interventions. 2018 Jan 22;11(2):145–57.
- Yohann B, Christophe de M de R, Gagandeep C, Dan R, Khadija B, Camille T, et al. Relationship Between Left Ventricular Ejection Fraction and Mortality in Asymptomatic and Minimally Symptomatic Patients With Severe Aortic Stenosis. JACC: Cardiovascular Imaging [Internet]. 2019 Jan 1;12(1):38–48. Available from: https://doi.org/10.1016/j.jcmg.2018.07.029
- Quere J-P, Monin J-L, Levy F, Petit H, Baleynaud S, Chauvel C, et al. Influence of Preoperative Left Ventricular Contractile Reserve on Postoperative Ejection Fraction in Low-Gradient Aortic Stenosis. 2006 [cited 2021 Nov 13]; Available from: http://www.circulationaha.org
- Ito S, Miranda WR, Nkomo VT, Connolly HM, Pislaru S v., Greason KL, et al. Reduced Left Ventricular Ejection Fraction in Patients With Aortic Stenosis. Journal of the American College of Cardiology. 2018 Mar 27;71(12):1313–21.
- Sevilla T, Revilla-Orodea A, San Román JA. Timing of Intervention in Asymptomatic Patients with Aortic Stenosis. European Cardiology Review [Internet]. 2021 Sep 3;16. Available from: https://www.ecrjournal.com/articleindex/ecr.2021.11
- Patel J, Rikhi R, Hussain M, Ayoub C, Klein A, Collier P, et al. Global longitudinal strain is a better metric than left ventricular ejection fraction: lessons learned from cancer therapeutic-related cardiac dysfunction. Current opinion in cardiology [Internet]. 2020 Mar 1 [cited 2021 Nov 13];35(2):170–7. Available from: https://pubmed.ncbi.nlm.nih.gov/31850935/
- Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiology [Internet]. 2018 Sep 1 [cited 2021 Nov 13];3(9):839. Available from: /pmc/articles/PMC6233650/
- Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. European Heart Journal [Internet]. 2017 Dec 1 [cited 2021 Oct 23];38(45):3351–8. Available from: https://academic.oup.com/eurheartj/article/38/45/3351/4002776
- Lindman BR, Dweck MR, Lancellotti P, Généreux P, Piérard LA, O’Gara PT, et al. Management of Asymptomatic Severe Aortic Stenosis: Evolving Concepts in Timing of Valve Replacement. JACC: Cardiovascular Imaging [Internet]. 2020 Feb 1 [cited 2021 Nov 13];13(2):481–93. Available from: https://doi.org/10.1016/j.jcmg.2019.01.036
- Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiology [Internet]. 2018 Sep 1 [cited 2021 Oct 8];3(9):839–47. Available from: https://jamanetwork.com/journals/jamacardiology/fu llarticle/2697026
- Magne J, Cosyns B, Popescu BA, Carstensen HG, Dahl J, Desai MY, et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC: Cardiovascular Imaging. 2019 Jan 1;12(1):84–92.
- Sengupta PP, Shrestha S, Kagiyama N, Hamirani Y, Kulkarni H, Yanamala N, et al. A Machine-Learning Framework to Identify Distinct Phenotypes of Aortic Stenosis Severity. JACC: Cardiovascular Imaging [Internet]. 2021 Sep;14(9):1707–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S193687 8X21002862
- Saeed S, Rajani R, Seifert R, Parkin D, Chambers JB. Exercise testing in patients with asymptomatic moderate or severe aortic stenosis. Heart [Internet]. 2018 Nov 1 [cited 2021 Oct 23];104(22):1836–42. Available from: https://heart.bmj.com/content/104/22/1836
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-Analysis of Prognostic Value of Stress Testing in Patients With Asymptomatic Severe Aortic Stenosis. The American Journal of Cardiology. 2009 Oct 1;104(7):972–7.
- Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective Study of Asymptomatic Valvular Aortic Stenosis. Circulation [Internet]. 1997 May 6 [cited 2021 Oct 23];95(9):2262–70. Available from: https://www.ahajournals.org/doi/abs/10.1161/01.CI R.95.9.2262
- Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients With Aortic Stenosis. Journal of the American College of Cardiology [Internet]. 2018 Feb 27 [cited 2021 Oct 23];71(8):860. Available from: /pmc/articles/PMC5821681/
- Bohbot Y, Renard C, Manrique A, Levy F, Maréchaux S, Gerber BL, et al. Usefulness of Cardiac Magnetic Resonance Imaging in Aortic Stenosis. Circulation: Cardiovascular Imaging [Internet]. 2020 May 1 [cited 2021 Nov 13];13(5):10356. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCI MAGING.119.010356
- Adriaans BP, Westenberg JJM, van Cauteren YJM, Gerretsen S, Elbaz MSM, Bekkers SCAM, et al. Clinical assessment of aortic valve stenosis: Comparison between 4D flow MRI and transthoracic echocardiography. Journal of Magnetic Resonance Imaging [Internet]. 2020 Feb 1 [cited 2021 Nov 13];51(2):472–80. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/jmri.26847
- Woldendorp K, Bannon PG, Grieve SM. Evaluation of aortic stenosis using cardiovascular magnetic resonance: A systematic review & meta-analysis. Journal of Cardiovascular Magnetic Resonance [Internet]. 2020 Jun 15 [cited 2021 Nov 13];22(1):1–9. Available from: https://jcmr-online.biomedcentral.com/articles/10.1186/s12968-020-00633-z
- R B, RJ E, C T, S S, S L, R H, et al. Rationale and design of the randomized, controlled Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) trial. American heart journal [Internet]. 2019 Jun 1 [cited 2021 Oct 24];212:91–100. Available from: https://pubmed.ncbi.nlm.nih.gov/30978556/
- Balciunaite G, Skorniakov V, Rimkus A, Zaremba T, Palionis D, Valeviciene N, et al. Prevalence and prognostic value of late gadolinium enhancement on CMR in aortic stenosis: meta-analysis. European Radiology [Internet]. 2020 Jan 1 [cited 2021 Nov 13];30(1):640–51. Available from: https://link.springer.com/article/10.1007/s00330-019-06386-3
- Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, et al. Myocardial scar and mortality in severe aortic stenosis: Data from the BSCMR Valve Consortium. Circulation [Internet]. 2018 Oct 30 [cited 2021 Nov 13];138(18):1935–47. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRC ULATIONAHA.117.032839
- Singh A, Chan DCS, Kanagala P, Hogrefe K, Kelly DJ, Khoo JP, et al. Short-term adverse remodeling progression in asymptomatic aortic stenosis. European Radiology [Internet]. 2021 Jun 1 [cited 2021 Nov 13];31(6):3923–30. Available from:
https://link.springer.com/article/10.1007/s00330-020-07462-9 - Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC: Cardiovascular Imaging. 2017 Nov 1;10(11):1320–33.
- Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee W, et al. Noncontrast Myocardial T1 Mapping by Cardiac Magnetic Resonance Predicts Outcome in Patients With Aortic Stenosis. JACC: Cardiovascular Imaging. 2018 Jul 1;11(7):974–83.
- Barone-Rochette G, Piérard S, de Meester De Ravenstein C, Seldrum S, Melchior J, Maes F, et al. Prognostic Significance of LGE by CMR in Aortic Stenosis Patients Undergoing Valve Replacement. Journal of the American College of Cardiology. 2014 Jul 15;64(2):144–54.
- Everett RJ, Tastet L, Clavel M-A, Chin CWL, Capoulade R, Vassiliou VS, et al. Progression of Hypertrophy and Myocardial Fibrosis in Aortic Stenosis. Circulation: Cardiovascular Imaging [Internet]. 2018 Jun 1 [cited 2021 Oct 23];11(6):7451. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCIMAGING.117.007451
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of Outcome in Severe, Asymptomatic Aortic Stenosis. http://dx.doi.org/101056/NEJM200008313430903 [Internet]. 2009 Aug 20 [cited 2021 Oct 23];343(9):611–7. Available from: https://www.nejm.org/doi/10.1056/NEJM200008313430903
- Pawade T, Clavel M-A, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, et al. Computed Tomography Aortic Valve Calcium Scoring in Patients With Aortic Stenosis. Circulation: Cardiovascular Imaging [Internet]. 2018 Mar 1 [cited 2021 Oct 23];11(3). Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCIMAGING.117.007146
- Utsunomiya H, Yamamoto H, Kitagawa T, Kunita E, Urabe Y, Tsushima H, et al. Incremental prognostic value of cardiac computed tomography angiography in asymptomatic aortic stenosis: Significance of aortic valve calcium score. International Journal of Cardiology. 2013 Oct 15;168(6):5205–11.
- Feuchtner GM, Müller S, Grander W, Alber HF, Bartel T, Friedrich GJ, et al. Aortic valve calcification as quantified with multislice computed tomography predicts short-term clinical outcome in patients with asymptomatic aortic stenosis. The Journal of heart valve disease. 2006 Jul;15(4).
- Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, et al. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. Journal of the American College of Cardiology [Internet]. 2014 May 20 [cited 2021 Oct 24];63(19):2016–25. Available from: http://dx.doi.org/10.1016/j.jacc.2014.02.581
- Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, et al. Natriuretic Peptides Predict Symptom-Free Survival and Postoperative Outcome in Severe Aortic Stenosis. Circulation [Internet]. 2004 May 18 [cited 2021 Oct 24];109(19):2302–8. Available from: https://www.ahajournals.org/doi/abs/10.1161/01.CIR.0000126825.50903.18
- Takagi H, Hari Y, Kawai N, Kuno T, Ando T. Meta-Analysis of Impact of Baseline N-TerminalPro-Brain Natriuretic Peptide Levels on SurvivalAfter Transcatheter Aortic Valve Implantation for Aortic Stenosis. The American Journal of Cardiology. 2019 Mar 1;123(5):820–6.
- EARLY TAVR: Evaluation of TAVR Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis - Full Text View - ClinicalTrials.gov [Internet]. [cited 2021 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT03042104
- Banovic M, Iung B, Bartunek J, Penicka M, Vanderheyden M, Casselman F, et al. The Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): A protocol update. American Heart Journal. 2018 Jan 1;195:153–4.
- Danish National Randomized Study on Early Aortic Valve Replacement in Patients With Asymptomatic Severe Aortic Stenosis - Full Text View - ClinicalTrials.gov [Internet]. [cited 2021 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT03972644
- The Early Valve Replacement in Severe ASYmptomatic Aortic Stenosis Study - Full Text View - ClinicalTrials.gov [Internet]. [cited 2021 Oct 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT04204915
Community Events Calendar


