Artificial Intelligence (AI) – potential benefits and challenges in Coronary Artery Disease – Focus on Diagnostic Imaging
| Take Home Messages |
|---|
|
Introduction
The field of AI is developing rapidly, especially in healthcare. A current Google search for “AI in health and social care” for example reaps 351 million hits1. Current examples of the literature include the Royal College of Physicians (RCP) official statement on AI in 2018, urging industry to address real-world challenges, doctors to scrutinize the technology and regulators to improve guidance and evaluation methods to assess AI2. Eric Topol (a pioneer of individualised medicine), in his book ‘Deep Medicine: How artificial intelligence can make healthcare human again’, explains how pattern recognition and machine learning can be used by doctors to better manage health and improve patient safety through home monitoring3. He also outlines how the connection between patients and doctors can be improved by enabling automated tasks and thereby freeing medical professionals to focus on providing care to patients.
Modern history of AI begins in the 1950s. Alan Turing (1956) published a landmark paper in which he proposed that it would be possible to create a machine that thinks4. The term “artificial intelligence” (AI) was first used by John McCarthy in 1956 at a Dartmouth Conference where the theme of the conference was “every aspect of learning or any other feature of intelligence can be so precisely described that a machine can be made to simulate it”5.
AI is a general term that encompasses any computerised programme that simulates characteristics of human intellect such as problem solving and learning. AI includes machine learning (ML) which is concerned with the automated discovery of statistical patterns in data without using explicit instructions. Two important criteria for ML to function are that data is detailed enough to answer the question being asked; and also that a ML technique is appropriate for the type, amount and complexity of the information available6.
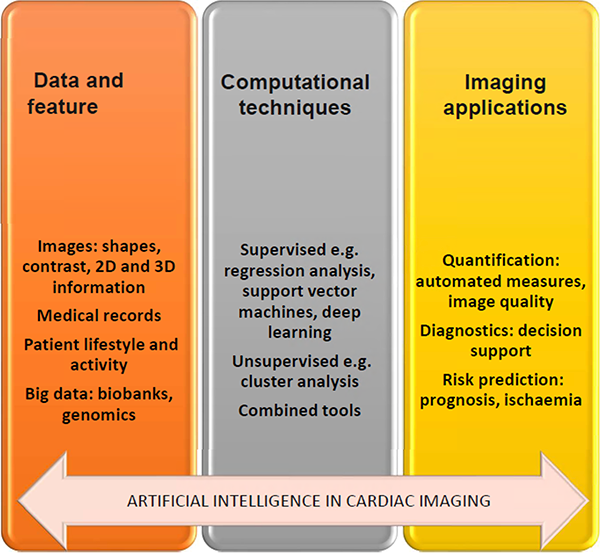
Figure 1: Steps to use artificial intelligence in cardiac imaging, modified from Dey et al6
There are various ways in which an algorithm can model a problem based on its interaction with the experience or the environment it is merged into. The two main learning styles used in machine learning are:
a. Supervised learning. Input data has a known ‘ground truth’ and is labelled, e.g. cardiac images with expert hand-drawn contours, and the model is prepared through a training process in which it is required to make predictions and is corrected when those predictions are flawed. This training process goes on until the model reaches an optimal level of accuracy on the training data7. A practical application of supervised learning is weather forecasting for accurate weather prediction. In cardiology, supervised learning techniques such as regression models have been used traditionally in risk prediction such as predicting the 30-day mortality risk for patients with ST-elevation myocardial infarction (TIMI)8. In cardiac imaging, automated segmentation of endocardial borders in unseen datasets is a common design goal of ML in cardiac MRI9.
b. Unsupervised learning. Input data is not labelled and does not have a known result. The model is prepared by deducing structures present within the input data – this may be through a mathematical process to systematically reduce redundancy, or it may be to organize data by similarity7. Cluster analysis is an unsupervised ML technique which provides a process of crafting homogeneous groups of data from hidden patterns in data without prior knowledge6. Examples of real world applications of unsupervised learning include genetics, where clustering DNA patterns are used to analyse evolutionary biology10, and cardiac imaging, where clustering has been used in the echocardiographic assessment of left ventricular function6. Deep learning (DL) is a subtype of unsupervised ML that uses artificial neural networks with multiple layers to learn directly from data. In DL, expert hand-drawn contours are not required up front. A convolutional neural network is an example of a DL network often used for image analysis tasks.
Significant milestones have been achieved using AI in various clinical fields such as ophthalmology (retinal imaging), pathology (image analysis) and dermatology (differentiation of benign vs malignant skin lesions). In the field of cardiology, AI technologies are being applied in clinical prediction, for example TIMI risk prediction as discussed above, cardiac imaging analysis, and home monitoring in the form of smartwatches which may be used for arrhythmia detection. According to a survey conducted by Attest, in 2019, 37.6% of millennials claimed to own such a device11. The reported benefits of smartwatch monitoring include self-management of chronic conditions such as Parkinson’s disease12 where smartwatches can be used to monitor gait abnormalities, facial tremors as well as speech, epilepsy whereby smartwatches can be used to detect tonic clonic seizure activity13, and weight management by monitoring eating behaviour14.
Coronary artery disease (CAD) represents the most common form of cardiovascular disease and is the greatest cause of mortality worldwide as well as accounting for more than 100,000 hospital admissions attributable to myocardial infarction in the UK each year15. By the very nature of its complexity and prevalence, coronary disease presents an ongoing opportunity for AI development. With the growing emphasis on precision, personalised medicine, which relies heavily on efficient, accurate diagnostic tools, cardiovascular imaging is likely to be a key area where the impact of AI will be noted. I therefore examine a few applications of AI in diagnosing CAD, with an emphasis on non-invasive imaging.
Use of AI in Coronary Artery Diagnostic Imaging Modalities
Coronary CT
There are multiple examples of the use of ML in the field of coronary CT. In the CONFIRM registry, a large 5-year multicentre prospective registry16 involving 10,030 patients undergoing coronary computed tomographic angiography (CCTA), ML was used to combine CT as well as clinical data, including Framingham scores. Predictive classifiers for all-cause mortality were developed and showed that a ML-developed algorithm could predict 5-year all-cause mortality significantly better than existing clinical or CCTA metrics alone. An important limitation of this study was that although prospectively collected, the CONFIRM registry data used to derive the model was observational, and the impact of selection bias cannot be underestimated16. This is relevant as subsequent findings may not be representative of the broader population.
Another example of the use of ML in cardiac CT is from the MESA (Multi-ethnic study of atherosclerosis) study. In this study of 6,814 asymptomatic patients undergoing coronary artery calcium (CAC), ML utilised all available clinical and CT data (CAC score, CAC volume scores, as well as extracardiac CAC scores). A comparison of areas under the curve (AUCs) by receiver operator curves for clinical data alone, CAC Agatston score alone, and the combination of all clinical and CT variables by ML showed that ML using all available clinical and non-contrast CT variables was superior to clinical risk factors and CAC score in predicting both coronary heart disease and cardiovascular disease events17.
Echocardiography
Echocardiography is at the forefront of diagnostics in cardiology; and although the use of AI in echocardiography is still work in progress, several applications have been developed. For instance, in their study, Madani et al18 trained a convolutional neural network to recognize 15 standard echocardiographic views, using a training set of 200,000 images. The researchers demonstrated an accuracy of 91.7% compared to 79.4% for board certified echocardiographers classifying a subset of the same test images. A possible explanation for this discrepancy offered by the authors is that the occasional misclassifications of single images most often involved views that can look similar to the human eye, e.g. confusion of an apical three-chamber view for an apical twochamber view18. I would add that the humans were disadvantaged in these comparisons as they underperformed (understandably) when presented with 2D stills taken from cine loops, or when images underwent random vertical and/or horizontal flips. In addition to accuracy, other studies such as Knackstedt et al have showed that AI could enable reproducible analysis of left ventricular function as well as longitudinal strain in approximately 8 seconds18,19. Such rapid assessments in echocardiography could potentially save clinician time, and in the process increase the availability of echocardiogram appointments for patients, the caveats here being the reproducibility and accuracy of AI-driven assessments.
Myocardial perfusion imaging (MPI)
In a large study by Arsanjani et al20, 1,181 reststress Tc-sestamibi dual isotope MPI studies were examined. The authors found that computational integration of quantitative image measures and clinical data by ML improves the diagnostic performance of automatic MPI analysis to a level rivalling expert analysis. A later study21 sought to investigate if early revascularisation in patients with suspected CAD could be effectively predicted by integrating clinical data and quantitative features derived from MPI by ML, and found that an MLbased approach was comparable or better than experienced readers in predicting early revascularisation after MPI, and was significantly better than standalone measures of perfusion derived from MPI.
Cardiac MRI
Using a large-scale dataset from the UK Biobank consisting of 4,875 subjects, Bai et al22 proposed an automated method using fully convolutional networks for CMR image analysis, which was comparable to human experts, with automated and manual segmentation showing that the computerhuman difference was close to or even smaller than the human-human difference for all metrics22. The authors concluded that this could be a starting point for automated CMR analysis facilitated by machine learning, with the DL method proving fast and scalable, providing analysis of long and short axis images for one subject within seconds. Authors therefore proposed that this would in turn have the potential to assist clinicians in CMR image analysis and therefore reduce cost and improve work efficiency, facilitating large-population imaging studies.
In a larger study presented in 2019 at Euro-CMR, Aung et al assessed the usefulness of DL and AI in the measurement of ventricular volumes, mass, and ejection fraction. The study demonstrated that DL could significantly shorten post-processing time (from 5000 studies in 7 months to 15,000 studies in <1 week). 28 genes (including titin and BAG3) were identified as statistically associated with certain patterns (‘morphotypes’) of left ventricular volumes, mass and ejection fraction. The authors also created a model to predict the risk of developing heart failure based on genetic information, sex, height, body mass index, blood pressure, dyslipidaemia, and tobacco and alcohol use23.
Benefits and promises
As noted above, AI provides key innovative applications in diagnosing coronary disease. These advantages could potentially translate into increased speed, accuracy, and test volumes, reduced human expert time and in certain cases enhanced patient
safety e.g. CT-FFR precluding the need for invasive coronary angiography. The caveat is that much work in this field still requires cross-validation and comparison with traditional methods before it can be fully and widely implemented.
Conclusion
The use of AI in cardiovascular imaging may help address challenges in throughput, efficiency, consistency, and accuracy which can happen at any stage of the diagnostic imaging chain. The key area where AI will pertain in imaging will be diagnostics, through image interpretation and disease characterization. However, the amalgamation of big data from imaging with the huge amounts of data from health records will – some hope – facilitate the delivery of highly personalised medicine in future. However, due consideration needs to be given to potential issues arising in the development of AI in imaging, namely training provision for imaging specialists, as well as the medicolegal issues related to ‘incorrect’ automatic segmentation of, for example, cardiac MRI datasets. Physicians will remain responsible for their clinical decisions, even if these were supported at key steps by an AI. Expert physicians should also feel empowered to question the results an AI gives, but this will not be possible without an understanding of how the AI-dependent algorithm was trained and in what population. Therefore , a redrawing of physician education and a holistic approach looking at the individual and socioeconomic characteristics of each patient, as compared with the training population, needs to be emphasized, so that AI only adds intelligent precision tools to enhance human clinical decisions, without replacing them6.
Disclosures
None.
Bibliography
- Fenech M, Buston O. AI in Cardiac Imaging: A UK-Based Perspective on Addressing the Ethical, Social, and Political
Challenges. Front Cardiovasc Med. 2020;7:54. - Physicians R college of. Artificial intelligence (AI) in health [Internet]. 2018. Available from: https://www.rcplondon.ac.uk/projects/outputs/artificialintelligence-ai-health
- News. Eric Topol pens book on artificial intelligence in medicine [Internet]. Scripps Research. 2019. Available from: https://www.scripps.edu/news-and-events/pressroom/2019/20190312-topol-deep-medicine.html
- Turing AM. Computing machinery and intelligence. Mind [Internet]. 1950 Oct 1;(236):433–60. Available from:
https://doi.org/10.1093/mind/LIX.236.433 - McCarthy J, Minsky ML, Rochester N, Shannon CE. A Proposal for the Dartmouth Summer Research Project on Artificial Intelligence, August 31, 1955. AI Mag [Internet]. 2006 Dec 15;27(4 SE-Articles). Available from:
https://www.aaai.org/ojs/index.php/aimagazine/article/view/1904 - Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, et al. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(11):1317–35.
- Jason Brownlee. A tour of machine learning algorithm. Machine learning mastery. 2020.
- Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J [Internet]. 2017 Jun 14;38(23):1805–14. Available from: https://doi.org/10.1093/eurheartj/ehw302
- Leiner T, Rueckert D, Suinesiaputra A, Baeßler B, Nezafat R, Išgum I, et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson [Internet]. 2019;21(1):61. Available from: https://doi.org/10.1186/s12968-019-0575-y
- Heidmann L. Unsupervised Machine Learning: Use Cases & Examples. 2020.
- Statista. PREMIUM Share of respondents who own a smart watch/health-tracker in UK 2019, by generation Published by Statista Research Department, Jan 22, 2021 According to a survey conducted by Attest, in 2019, millennials are more likely than members of any other g [Internet]. 2021. Available from: https://www.statista.com/statistics/1044033/uksmartwatch-health-trackert-ownership/
- Sharma V, Mankodiya K, De La Torre F, Zhang A, Ryan N, Ton TGN, et al. Spark: Personalized parkinson disease interventions through synergy between a smartphone and a smartwatch. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics). 2014;103–14.
- Lockman J, Fisher RS, Olson DM. Detection of seizure-like movements using a wrist accelerometer. Epilepsy Behav [Internet]. 2011 Apr 1;20(4):638–41. Available from: https://doi.org/10.1016/j.yebeh.2011.01.019
- Kalantarian H, Sarrafzadeh M. Audio-based detection and evaluation of eating behavior using the smartwatch platform. Comput Biol Med [Internet]. 2015;65:1–9. Available from: https://www.sciencedirect.com/science/article/pii/S0010482515002553
- British Heart foundation. BHF statistics factsheet- UK. 2020; Available from: https://www.bhf.org.uk/what-we-do/ourresearch/heart-statistic
- Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, et al. Machine learning for prediction of allcause mortality in patients with suspected coronary artery disease: A 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38(7):500–7.
- Nakanishi R, Dey D, Commandeur F, Slomka P, Betancur J, Gransar H, et al. Machine Learning in Predicting Coronary Heart Disease and Cardiovascular Disease Events: Results From the Multi-Ethnic Study of Atherosclerosis (Mesa). J Am Coll Cardiol [Internet]. 2018;71(11):A1483. Available from: http://dx.doi.org/10.1016/S0735-1097(18)32024-2
- Madani A, Arnaout R, Mofrad M, Arnaout R. Fast and accurate view classification of echocardiograms using deep learning. npj Digit Med [Internet]. 2018;1(1):6. Available from: https://doi.org/10.1038/s41746-017-0013-1
- Knackstedt C, Bekkers SC, Schummers G, Schreckenberg M, Muraru D, Badano LP, et al. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: the FAST-EFs multicenter study. J Am Coll Cardiol. 2015;66:1456–66.
- Arsanjani R, Xu Y, Dey D, Vahistha V, Shalev A, Nakanishi R, et al. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J Nucl Cardiol. 2013;20(4):553–62.
- Arsanjani R, Dey D, Shalev A, Khachatryan T, Hayes S, Fish M, et al. Improved Accuracy of Myocardial Perfusion Spect for Prediction of Revascularization By Machine Learning in a Large Population. J Am Coll Cardiol. 2014;63(12):A1229.
- Bai W, Sinclair M, Tarroni G, Oktay O, Rajchl M, Vaillant G, et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson. 2018;20(1):1–12.
- Aung N. Genetic architecture of left ventricular phenotypes derived from 17,000 CMR studies in the UK Biobank population imaging cohort. Young Investigator Award session, EuroCMR. 2019.
Community Events Calendar


