An ancient remedy with a modern twist: colchicine in cardiovascular disease
| Take Home Messages |
|---|
|
Introduction
Colchicine is an ancient remedy with anti-inflammatory properties that have proved useful throughout the history of medicine. It is derived from the Colchicum Autumnale (Autumn Crocus) plant (Figure 1), native to the British Isles and also found in mainland Europe. The first description of its use is seen in the Ebers papyrus of ancient Egypt in 1550 BC for treatment of pain and swelling1, and today colchicine remains a safe and low-cost drug (£8.18/100 tablets)2 used in the treatment of gout, Familial Mediterranean Fever and pericarditis.
Over the years, the heart as an “immune organ” has become an increasingly popular notion, with the discovery of multiple complex inflammatory cascades involved in its response to injury3.
In fact, since the 1990s we have known that inflammation plays a pivotal role in atherogenesis and myocardial infarction (MI)4. Thus, over the last 2 decades therapeutic approaches to improve outcomes of patients with cardiovascular disease have naturally turned towards anti-inflammatory therapies, though with variable clinical success. The CANTOS trial5 was the first clinical trial to support the inflammatory hypothesis of atherothrombosis and showed that canakinumab, a monoclonal antibody targeting IL-1β, was effective at preventing adverse cardiac events over a median of 3.7 years in patients with a history of MI and high C-reactive protein. Unfortunately, its drawback was an association with a significant rise in fatal infections. More recently, the CIRT trial, which looked at the safety and benefit of low-dose methotrexate in patients with stable coronary artery disease (CAD), failed to show any significant reduction in major adverse cardiovascular events over a median period of 2.7 years, and again resulted in increased risk of significant infection, as well as leucopenia, anaemia and transaminitis6. The challenge to identify safe and effective anti-inflammatory therapies likely reflects the convoluted and highly complex inflammatory pathways involved in atherogenesis, and the difficulties in differentiating those which have influence on the clinical endpoint from those which do not and are simply by-standing biomarkers of atherogenesis. These studies also highlight problems with the translation of work in animal models to humans, where inflammatory and ischaemic processes in animals do not completely mimic those in humans7.
Over the last few years, a spotlight has been shone on colchicine as a promising contender, with initial studies revealing its beneficial effects in reducing bare metal stent re-stenosis in diabetic patients8, decreasing infarct size in ST-elevation MI9 and significantly reducing rates of post-pericardiotomy syndrome and post-operative atrial fibrillation following cardiac surgery10. Here I will re-visit the pharmacological mechanisms of colchicine before summarising three of the latest studies lending further support to its beneficial effects, specifically in the management of acute and chronic coronary disease and malignant pericardial effusions.

Figure 1: Autumn Crocus plant11
Colchicine: Mechanisms of action
Colchicine exerts its anti-inflammatory effects via multiple pathways (Figure 2). It binds to tubulin in cells and impairs the function of microtubules12, thereby interfering with important mechanisms of inflammatory cell activity, namely cell division, cell migration and cytokine release13. Colchicine also inhibits the expression of selectins by neutrophils and endothelial cells thus inhibiting neutrophil migration and adhesion to the inflamed endothelium14. More recently, colchicine has been found to inhibit the NLRP3 inflammasome, a complex of intracellular proteins assembled and activated in inflammatory states, and key in producing the highly inflammatory and atherogenic cytokines IL-1β and IL-1815. Studies have shown a strong correlation between levels of IL-1β and the extent of atherosclerosis within coronary arteries taken from patients with ischaemic heart disease16,15, as well as levels of IL-18 and cardiovascular death in stable and unstable angina17,15.
Colchicine is absorbed in the jejunum and ileum, with peak plasma concentrations achieved 1-2 hours after ingestion and maximal anti-inflammatory effects after 24 hours. It is excreted via the hepatobiliary and urinary systems and is thus largely safe in patients with normal hepatic and renal function. The most common side effects are gastrointestinal (nausea, vomiting, diarrhoea), occurring in 5-10% of patients, and usually self-limiting (18). More toxic effects can occur with excessive doses or concomitant use of cytochrome P450 inhibitors and include myelosuppression, acute renal and hepatic failure, peripheral neuropathy, ascending paralysis, seizures and respiratory failure18.
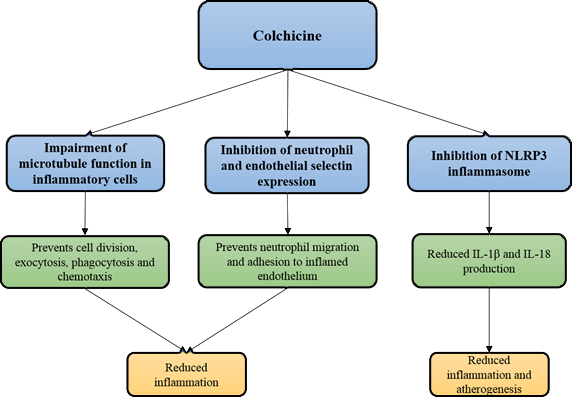
Figure 2: The mechanisms of action of Colchicine in the prevention of inflammation and atherogenesis
Study one: Colchicine in Chronic Coronary Disease – The LoDoCo2 Trial
The LoDoCo2 trial was a randomised, controlled, double-blind trial involving 13 centres in Western Australia and 30 centres in the Netherlands between 2016 and 201819. It came after the LoDoCo trial, a prospective randomised single-blinded study published in 2012, had shown that colchicine was effective in preventing cardiovascular events in patients with stable coronary disease, but was criticised for its single-blinded methodology and small sample size (n=282 colchicine group, n=250 placebo group)20. In the LoDoCo2 trial, authors attempted to establish whether 0.5 mg of colchicine once daily (OD) led to a reduction in cardiovascular events in patients with chronic coronary disease. Eligible patients were aged 35-82 with any evidence of coronary disease on invasive angiography or CT angiography, or a coronary artery calcium score of at least 400 Agatston units on a coronary artery calcium scan, and had been clinically stable for at least 6 months. Exclusion criteria were moderate-to-severe renal impairment, severe heart failure, severe valvular heart disease or known side effects from colchicine.
5522 patients (mean age 66) were randomly assigned to receive colchicine 0.5 mg OD (n=2762) or placebo (n=2760). Notably, the majority of patients were already taking evidence-based secondary prevention therapies such antiplatelets or anticoagulants (99.7%), lipid-lowering agents (96.6%), beta-blockers (62.1%) and renin-angiotensin inhibitors (71.7%). The primary end point was a composite of cardiovascular death, spontaneous (non-procedural) MI, ischaemic stroke or ischaemia-driven coronary revascularisation. The key secondary end point was a composite of cardiovascular death, spontaneous MI or ischaemic stroke. Patients were followed up for a median of 28.6 months.
The incidence of a composite of cardiovascular death, spontaneous MI, ischaemic stroke and ischaemia-driven revascularisation was significantly lower in the colchicine group (n=187; 6.8%) vs. placebo group (n=264; 9.6%; hazard ratio (HR) 0.69; confidence interval (CI) 0.57 – 0.83; p<0.001). The key secondary composite end-point also occurred in significantly fewer patients in the colchicine group (n=115; 4.2%) vs. the placebo group (n=157; 5.7%; HR 0.72; CI 0.57-0.92; p=0.007), and these effects were consistent across subgroup analyses, including sex, age (>65 years vs. <65 years), smoking status, hypertension, diabetes, renal function, prior acute coronary syndrome and prior coronary revascularisation.
The reported benefits of colchicine appear striking, but several study limitations must also be considered. Firstly, caution must be exercised when attempting to generalise results to women, ethnic minority patients and those with renal impairment. There were a lower-than-expected number of women in the study (n=846; 15.3%), which may reflect the longstanding challenge of enrolling women in cardiovascular clinical trials, often linked to underestimating cardiac risk21 and misinterpretation of symptoms of CAD in this population22.
The authors also do not report on outcomes by ethnicity, and yet we know that the risk of developing CAD is much higher in the South Asian population23, and that 14% of the population in the UK are within the black and ethnic minority group24.
Another group of patients to which these results need to be applied with care are those with renal impairment. Colchicine may be used with caution (at lower doses or greater dosing intervals) in patients with renal dysfunction as long as the eGFR is above 1025. However, in LoDoCo2 all patients with moderate to severe renal impairment were excluded and the vast majority of those included had an eGFR of 60 or above (94.4%), meaning that application of results to those with higher degrees of renal impairment should be exercised with a degree of caution.
A further limitation is the drop-out rate during the open-label run-in period, which was conducted to identify patients who were stable and could tolerate colchicine at the trial dose. It is interesting to see that 15.4% of patients dropped out of the trial at this stage due to side effects or perceived side effects, the most common being gastrointestinal upset, highlighting that tolerability of the drug may preclude its real-world long-term use.
Finally, there was a higher non-cardiovascular death rate among the colchicine group (HR 1.51; CI 0.99-2.31), though the causes of death were not consistent and there was no significant difference in rates of cancer diagnosis, hospitalisation for pneumonia, infection or gastrointestinal symptoms between the trial groups. We know that these are not the only causes of morbidity and mortality when considering colchicine toxicity, and data on concomitant cytochrome P450 inhibitor use, a known risk factor for toxicity, as well as liver toxicity rates may have helped to shed light on the reasons for this unexpected observation. There are a number of randomised controlled studies into colchicine’s effects on atrial fibrillation currently underway (COP-AF (Colchicine For the Prevention Of Perioperative Atrial Fibrillation In Patients Undergoing Thoracic Surgery); NCT03310125 and END-AFLD (Effect of Low Dose ColchiciNe on the InciDence of POAF); NCT03015831) and the non-cardiovascular death rates within these studies will be of particular interest in exploring concerns regarding the safety of colchicine raised in this study.
There were a number of positive aspects to the study that increase the chance of it translating to real world practice. These include the low loss to follow-up rates which were equal in both groups (n=289; 10.5% colchicine, n=291; 10/5% placebo), as well as the fact that colchicine is a cheap and already widely available drug that could be quickly and easily implemented into clinical practice. It is currently unclear how long the duration of colchicine therapy in such patients would be, but potentially long term, and so longer-term data may be required before such implementation could go ahead.
All being considered, these results show that colchicine, in addition to proven secondary prevention therapies, significantly reduces the composite occurrence of cardiovascular death, MI, stroke and ischaemia-driven revascularisation in patients with stable CAD.
Study two: Colchicine after Myocardial Infarction – The Colchicine Cardiovascular Outcomes Trial (COLCOT)
The COLCOT trial was a randomised double-blind placebo-controlled trial involving 167 centres in 12 countries from 2015 to 2018, and was designed to determine the effects of 0.5mg OD colchicine on cardiovascular outcomes in patients who had recently had an MI26. Eligible patients had suffered an MI within 30 days before enrolment, had completed any planned percutaneous revascularisation procedures and were receiving treatment according to national guidelines. Exclusion criteria included severe heart failure, left ventricular ejection fraction less than 35%, stroke within the previous 3 months, a type 2 index MI, coronary bypass surgery either planned or within the previous 3 years, severe renal disease and severe hepatic disease.
The primary efficacy end point was a composite of death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalisation for angina leading to coronary revascularisation. The secondary end points consisted of the components of the primary efficacy end point.
4745 patients (mean age 60.6; 19.2% female) were randomised to either colchicine 0.5mg OD (n=2366) or placebo (n=2379) and were followed up for a median of 22.6 months. Patients were enrolled at a mean of 13.5 days after MI and 93% were treated with percutaneous coronary intervention. The median duration of trial drug treatment was 19.6 months in the colchicine group and 19.5 months in the placebo group. The incidence of the primary composite efficacy end point was significantly lower in the colchicine group (n=131; 5.5%) vs. the placebo group (n=170; 7.1%; HR 0.77; CI 0.61 to 0.96; p=0.02), and remained so after adjustment for baseline covariates. This was predominantly due to a lower incidence of strokes (HR 0.26; CI 0.1-0.7) and urgent hospitalisations for angina leading to coronary revascularisation (HR 0.5; CI 0.31-0.81). There were no statistically significant differences in the incidence of serious adverse events, although there were increases in rates of pneumonia in the colchicine group.
Criticisms of the trial include a drop-out rate of just over 18% in both arms, higher than that in the LoDoCO2 trial, as well as a sample size not large enough to enable full analysis of each component of the composite end points, both of which reduce the trial’s ability to detect any real benefit or harm related to colchicine. The size of the treatment effect is also questionable when taking into consideration the trial’s fragility index. The fragility index is the minimum number of patients who must be moved from the non-event group to the event group to turn a significant result into a nonsignificant one27. The fragility index in this trial is 5, meaning that if 5 patients in the colchicine group moved from not having the primary endpoint to having it, then the statistical significance of the result is lost. The fragility indices for stroke and stable angina are 3 and 7 respectively, again very low, and particularly when there were a significantly higher number of patients (n=89) who were lost to follow-up. Altogether, this does reduce the impact of the trial’s conclusions about the efficacy of colchicine in MI.
Finally, looking at the adverse event rates related to colchicine, the authors report that there were no significant differences in overall adverse event rates, but that the number of cases of pneumonia was higher in the colchicine group. Closer attention to detail reveals that there were more than double the number of pneumonia cases in the colchicine group vs. placebo (n=21 vs. n=9 respectively, p=0.03), suggesting more significance than the authors give acknowledgement to and again raises concerns about the safety of colchicine.
Overall, the results reveal that in addition to percutaneous coronary intervention and current secondary prevention medications, colchicine significantly reduced the secondary endpoints of incidence of stroke and hospitalisation for angina requiring coronary revascularisation compared with placebo, however the sensitivity of the trial to detecting real benefit or harm related to colchicine and the robustness of the data is questionable and it does not look likely that colchicine will be a guideline recommendation in this patient population as yet.
Study three: Colchicine in patients with malignant pericardial effusion
This third and latest study published in JACC was a single-centre cohort study based in Korea, looking at the effect of anti-inflammatory drugs including colchicine on clinical outcomes in patients with malignant pericardial effusion28. Study participants were those with active cancer who had undergone pericardiocentesis for confirmed malignant pericardial effusion between 2007 and 2018. This was defined by the presence of malignant cells in the pericardial fluid or an exudative effusion with high lactate dehydrogenase and carcinoembryonic antigen levels in lung cancer patients, or evidence of direct pericardial invasion of the malignancy. Cancer patients who had been in remission for over 5 years or who had developed iatrogenic pericardial effusions were excluded. The duration of follow-up was 24 months after pericardiocentesis or until December 31st 2019, whichever came first. The primary outcome was a composite of all-cause death and re-pericardiocentesis or a pericardial window operation for recurrent effusion.
Pericardiocentesis and post-procedural care were performed according to the institution’s guidelines. This included an echo within 3 days post removal of the pericardial drain to check for pericardial adhesion, constriction or residual effusion. In those with evidence of constriction or pericardial adhesion, combinations of colchicine (0.6mg BD for 2 months), non-steroidal anti-inflammatory drugs (NSAIDs) (most commonly ibuprofen 600mg TDS, tapered after 2 weeks) and steroids (0.5mg/kg/day prednisolone or an equivalent) were then administered according to the responsible physician’s discretion. The duration of treatment was determined by the patient’s response at follow-up and the physician’s discretion.
445 patients were included in the study (median age 57 years; 55.7% male), of which 96.2% had advanced stage cancer. The most common cancers were lung cancer (63.4%), breast cancer (11%) and gastrointestinal cancer (7.4%). At 3 days post drain removal, echocardiography (n = 376) revealed pericardial adhesion in 70.7% of cases and pericardial constriction in 36.5%. Authors then divided patients who had had post-pericardiocentesis echo into those who had received colchicine and those who had not. A total of 91 (24.2%) patients took colchicine for a mean period of 63 days after pericardiocentesis. Evidence of pericardial adhesion (83.1% vs. 66.4%; p=0.003) and constriction (57.3% vs. 29.4%; p<0.001) post pericardiocentesis and prior to starting anti-inflammatory treatment were more frequent in the colchicine group, as was the use of NSAIDs (31.9% vs. 21.4%; p=0.04) and steroids (28.6% vs. 9.1%; p<0.001). The incidence of primary composite events was significantly lower in the colchicine group (112 per 100 patient-years) vs. the non-colchicine group (154 per 100 patient-years; p=0.03), which was maintained after multiple sensitivity analysis and adjustment for age, gender, cancer status, ongoing chemotherapy, and effusion size. There was also a significantly lower risk of all-cause death (HR 0.75; CI 0.58 to 0.98; p=0.03). Post hoc analyses performed to investigate whether there was an association between colchicine and clinical outcomes across various subgroups demonstrated a consistent effect of colchicine on lowering the risk of adverse events.
There are several limitations to this study. Firstly, this was a non-randomised, non-blinded observational study with no placebo-controlled group meaning that biases of selection, data collection and analysis could not be eliminated. This is likely to have resulted in a study population not entirely reflective of the general UK population, as well as variability in the chances of each study participant receiving any of the study drugs. For example, clinicians’ assessment of patient fitness and frailty, the severity of echo findings and perceived benefit of each anti-inflammatory drug are all highly likely to have influenced which patients did and did not receive colchicine. The decision to initiate an anti-inflammatory agent, the type of agent, the dose, duration and combination of agents used were all up to individual physicians’ discretion. We can see that patients with more severe findings at the echo post drainage (diffuse constriction or adhesion) were more likely to receive all 3 study drugs. It is not clear how the properties of each anti-inflammatory are affected when used in combination, but they could be synergistic with a more potent anti-inflammatory effect achieved with a combination and therefore give rise to a misleading association between colchicine and lower mortality rates.
It is also important to highlight that patients with multiple malignancy types and therefore different cancer treatments were included and it is difficult to know the impact of each cancer treatment on the pericardial effusion recurrence rate.
Lastly, the rates of adverse events are not reported in the study and it would have been useful to know the rates of infection in the colchicine vs. non-colchicine group, particularly in light of the findings in the previous two trials.
Nonetheless, the data presented shed light on the potential favourable effect of colchicine in significantly reducing effusion recurrence and mortality in active cancer patients with malignant pericardial effusion, and serve as a catalyst for prompting the necessary future prospective multi-centre randomised blinded placebo-controlled trials confirming these results and clarifying the optimal timing, dose and duration of colchicine.
Conclusion
Inflammation has long been implicated in the pathogenesis of various cardiac diseases, including CAD, hypertension and pericardial disease, to name but a few. Cell division, recruitment, adhesion and cytokine production are key mechanisms in the development and perpetuation of inflammation, and are potently inhibited by colchicine, a drug most known for its beneficial effects in gout, familial Mediterranean fever and pericarditis, until recently. Here I have summarised three recent studies which shed light on potential new uses for this cheap and widely available drug.
The LoDoCo2 trial demonstrated that colchicine significantly reduced the rate of ischaemic cardiovascular events in patients with stable coronary disease, and had well-matched baseline patient characteristics with low drop-out rates and good treatment effect. However, the applicability of results to certain population groups is limited and there was the unexpected finding of more non-cardiovascular deaths associated with colchicine. COLCOT reported reduced incidences of stroke and the need for coronary revascularisation for angina following acute MI with the use of colchicine, but the robustness of the overall reported treatment effect may be questioned with low subgroup patient numbers, higher drop-out rates than in LoDoCo2 and a low fragility score.
The different degrees of clinical benefit associated with colchicine in each of these studies may reflect the different inflammatory pathways and molecules involved in stable coronary disease vs. acute MI29, or that the same inflammatory mediators and pathways play different roles in each of these pathological processes.
Finally, colchicine also appears to reduce the need for recurrent pericardiocentesis in active cancer patients with malignant pericardial effusion which is likely to reduce hospital re-admission rates, length of stay and thus improve the quality of life of these patients. Having said that, this was an observational study without randomisation, blinding or placebo-control and so has multiple limitations. In the future, randomised controlled studies are required to determine the optimal timing, duration and dose of colchicine in active cancer patients with malignant pericardial effusion and we need clarity surrounding the higher non-cardiovascular death rates associated with colchicine observed in the LoDoCo2 trial.
I look forward to two much awaited studies, COP-AF and END-AFLD, which I hope will not only give us this sought-after clarification, but also reveal yet another cardiological role for colchicine in the prevention of post-operative atrial fibrillation, currently an area of contention with two previous trials revealing conflicting results (COPP (Colchicine for the Prevention of the Postpericardiotomy Syndrome Atrial Fibrillation Substudy; NCT00128427) and COPP-2 (COlchicine for Prevention of the Postpericardiotomy Syndrome and Postoperative Atrial Fibrillation; NCT01552187)).
Disclosures
None
References
- Nerlekar N , Beale A, Harper RW. Colchicine — a short history of an ancient drug. Med J Aust. 2014;201(11):687–8.
- https://bnf.nice.org.uk/medicinal-forms/colchicine.html [Accessed 05.11.2020]
- Epelman S., Liu P., Mann D. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15(2): 117-29
- Fioranelli M., Bottaccioli A., Bottaccioli F et al. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front Immunol. 2018;9: 2031
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-1131.
- Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752-762
- Rymer JA and Newby K. Failure to Launch: Targeting Inflammation in Acute Coronary Syndromes. JACC Basic Transl Sci. 2017:2(4):484-497
- Deftereos S, Giannopoulos G, Raisakis K, et al. Colchicine Treatment for the Prevention of Bare-Metal Stent Restensosis in Diabetic Patients. 2013: 61(16): 1679-1685
- Deftereos S, Giannopoulos G, Angelidis C, et al. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction. Circulation. 2015;132(15):1395-1403
- Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124(21):2290-2295
- https://wellcomecollection.org/works/tchu3783 [Accessed 05.11.2020].
- Andreu JM and Timasheff SN. Tubulin bound to colchicine forms polymers different from microtubules. Proc Natl Acad Sci USA. 1982;79(22):6753–6756
- Dalbeth N , Lauterio TJ and Wolfe HR. Mechanism of Action of Colchicine in the Treatment of Gout. Clin Ther. 2014;36:1465–79
- Cronstein BN., Molad Y., Reibman J et al. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96(2): 994-1002
- Martinez GJ., Celermajer DS and Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269: 262-271.
- Galea J, Armstrong J, Gadsdon P, et al. Interleukin-1 beta in coronary arteries of patients with ischaemic heart disease. Arterioscler. Thromb. Vasc. Biol. 1996; 16:1000-1006
- Blankenberg S, Tiret L, Bickel C, et al. Interleukin-18 is a strong predictor of cardiovascular death in sTable and unstable angina. Circulation. 2002; 106:24-30
- Angelidis C., Kotsialou Z., Kossyvakis C et al. Colchicine Pharmacokinetics and Mechanism of Action. Bentham Science. 2018; 24(6): 659-663
- Stefan M. Nidorf., Aernoud T.L. Fiolet., Arend Mosterd et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838-1847
- Nidorf SM., Eikelboom JW., Budgeon CA et al. Low-dose Colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2012;29;61(4):404-410
- Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005; 1;111(4):499-510
- Chiaramonte GR. Gender Bias in the Assessment of Coronary Heart Disease Gender Difference in Cardiovascular Device Trials. FDA White Oak Conference Center; 2008.
- Meadows TA, Bhatt DL, Cannon CP, et al. Ethnic differences in cardiovascular risks and mortality in atherothrombotic disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) registry. Mayo Clin Proc. 2011;86(10):960-967.
- https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest [Accessed 16.12.2020]
- https://bnf.nice.org.uk/drug/colchicine.html#renalImpairment [Accessed 16.12.2020]
- Tardif JC., Kouz S., Waters D et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505
- Narayan VM, Gandhi S, Chrouser K, et al. The fragility of statistically significant findings from randomised controlled trials in the urological literature.BJU Int. 2018; 122:160–166.
- Kim SR., Kim E K., Cho J et al. Effect of Anti-inflammatory Drugs on Clinical Outcomes in Patients With Malignant Pericardial Effusion. J Am Coll Cardiol. 2020;76 (13); 1551-61
- Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies Nat Rev Cardiol. 2017;14(3):133-144.
Community Events Calendar


