Radiation safety in the cath lab: does it still matter?
| Take Home Messages |
|---|
|
Introduction
The use of ionising radiation has become central to the modern management of patients, in particular those with a range of cardiovascular conditions. In 2010, it was estimated that 16% of UK population’s radiation exposure UK came from medical examinations and procedures,1 which equates to a lifetime risk of fatal cancer of 1 in 50,000.2 Given that cardiologists have been reported to be responsible for up to 40% of this dose,3 it is therefore critical that cardiologists understand the risks and have strategies for reducing the dose. However, it is not just patients who are exposed to ionising radiation; cardiologists and the cardiac catheterisation laboratory team are also exposed during procedures. Historically, the most active interventional cardiologists had exposures equating to an excess lifetime risk of cancer of 1 in 100.3 Despite the variation in the reported dose that medical staff receive, it appears to have dramatically declined over time.4 Whilst technological advances have resulted in improved image quality at a reduced dose, it is still paramount that cardiologists understand the risks to their patients and clinical staff as well as being adept at utilising strategies to reduce the risk resulting from ionising radiation. In this editorial I will aim to describe the risks of ionising radiation to both patients and staff as well as detailing strategies that the cardiologist can undertake to reduce the expose to both patients and staff.
Deterministic and stochastic effects
The consequences of exposure to ionising radiation can be categorised as either stochastic or deterministic (see Table 1).5,6 Deterministic effects occur once the received dose reaches a certain threshold (which is often specific to that tissue) and as such could be considered predictable. On the other hand, stochastic effects could be considered random as they result from deoxyribonucleic acid (DNA) damage without cell death and may eventually result in cell proliferation and subsequent neoplastic disease. Although there is no threshold for stochastic effects (i.e. these can occur after any degree of exposure) the risks of stochastic effects are proportional to the dose.5,6 The risk of stochastic effects is influenced by a number of factors including the age and gender of the individual (children and women are at higher risk)6 and the site exposed. Each body tissue group has a different sensitivity to the effects of ionising radiation (see Figure 1).
Table 1. Characteristics of deterministic and stochastic effects of ionising radiation (adapted from 6) | ||
|---|---|---|
Deterministic | Stochastic | |
Dose level | Medium to high | Any dose a |
Latency | Short | Long |
Threshold dose | Yes | Probably not |
Mechanism | Cell death | DNA damage without cell death |
a risk proportional to dose. DNA deoxyribonucleic nucleic acid. | ||
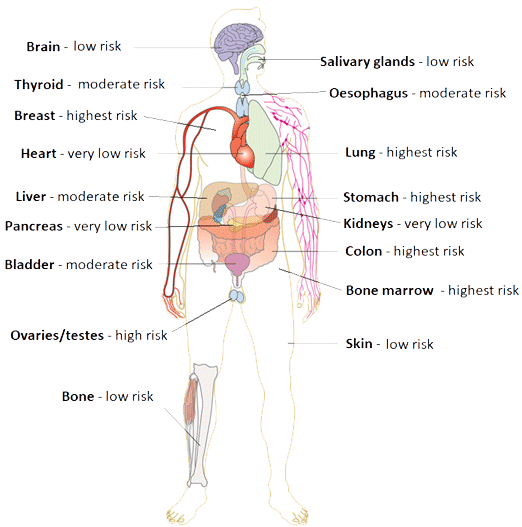
Figure 1. Relative organ sensitivity to ionising radiation when compared with the skina
arelative to very low risk: low risk 1.25x greater risk; moderate risk 5x greater risk; high risk 10x greater risk; highest risk 15x greater risk. Data from 6 image adapted using a creative commons licence.
Dose exposure parameters
In order to evaluate the risks, cardiologists need to have an understanding of the different parameters used to express the degree of radiation that the patient is exposed to. The fluoroscopic time measures the total time that fluoroscopy is used but, if considered alone, this is likely to underestimate the exposure because it does not take into account the acquisitions.5 The cumulative air kerma (Grays) measures the dose at a particular spot and is closely linked with the risk of deterministic effects.5 The dose area product (DAP) is the sum of the air kerma and the field of exposure. This provides a good estimate of the patient burden and provides an indication of the risk of stochastic effects.5
Patients’ perspective
Risks
Cardiac catheterisation laboratory lab procedures account for around 12% of all radiological examinations but 48% of the total dose received by patients.6 Table 2 demonstrates the relative exposure resulting from routine fluoroscopic cardiovascular procedures. The key concern when using ionising radiation is the risk of causing neoplastic transformation. In the UK, in 2010, medical radiation use was estimated to cause around 0.6% of all cancers.7 Given that these procedures have the potential to offer both symptomatic and prognostic benefits to patients, a considered approach of the risks involved should be undertaken. As such a discussion about the risks of ionising radiation should form part of the consent process for all fluoroscopic procedures. The relative risk will be based on patient factors (younger age being associated with higher risk and obesity likely to result in higher doses to achieve the same image quality) and procedural factors (including procedure type and complexity) (see Table 2).
Table 2. Average doses from standard cardiovascular fluoroscopic procedures and associated risk of malignancy for a 50 year old malea (adapted from 6,8) | |||
|---|---|---|---|
Procedure | Equivalent chest x-rays | Equivalent background radiation (years) | Additional lifetime risk of neoplasm |
Coronary angiography | 350 | 2.9 | 1/1,500 |
Percutaneous coronary intervention | 750 | 6.3 | 1/667 |
Transcutaneous aortic valve insertion b | 1,650 | 13.7 | 1/300 |
Diagnostic electrophysiology study | 160 | 1.2 | 1/3,000 |
Atrial fibrillation ablation | 830 | 6.9 | 1/600 |
Pacemaker | 200 | 1.6 | 1/2,500 |
Cardiac re-synchronisation device | 1,100 | 9.1 | 1/450 |
a note for females risk should be multiplied by 1.38 and halved for an 80 year old. b transfemoral. | |||
In terms of deterministic effects, skin erythema, temporary hair loss and skin burns all have a specific threshold above which the severity increases with increasing dose in a linear fashion, which relates to the complexity of the procedure and the body mass of a patient (with the obese patient requiring higher doses for the same quality of image).8 Skin injury can occur with 60 minutes of low dose fluoroscopy or 20 minutes of high dose fluoroscopy.3 Importantly the threshold above which deterministic effects occur will vary between patients.
How to reduce the dose
Whilst lower doses will reduce the overall risk of radiation induced complications, patients exposed to radiation will always carry a risk of stochastic effects; the only way to reduce a patient’s risk to zero is not to perform the procedure altogether. Measures to reduce the exposure dose have to be balanced with the need to ensure that the image quality is sufficient to allow diagnosis and treatment. Thus the mantra, As Low As Reasonably Achievable (ALARA) has been adopted.
A number of software and hardware developments have allowed a reduction in the dose received by the patient without altering the quality of the imaging.9 There are specific strategies that clinicians can employ to lower the dose that a patient receives (see Figure 2). The use of different angulations during a procedure means that the dose is spread over a wider area of skin thus reducing the risk of skin reactions. Whilst a specific view maybe important for the procedure, slight adjustments may allow a similar view but mean that a different area of skin is exposed. Furthermore using less steep angulation during the procedure will also reduce the dose, with posterior-anterior projection having the lowest dose and lateral carrying the highest dose.
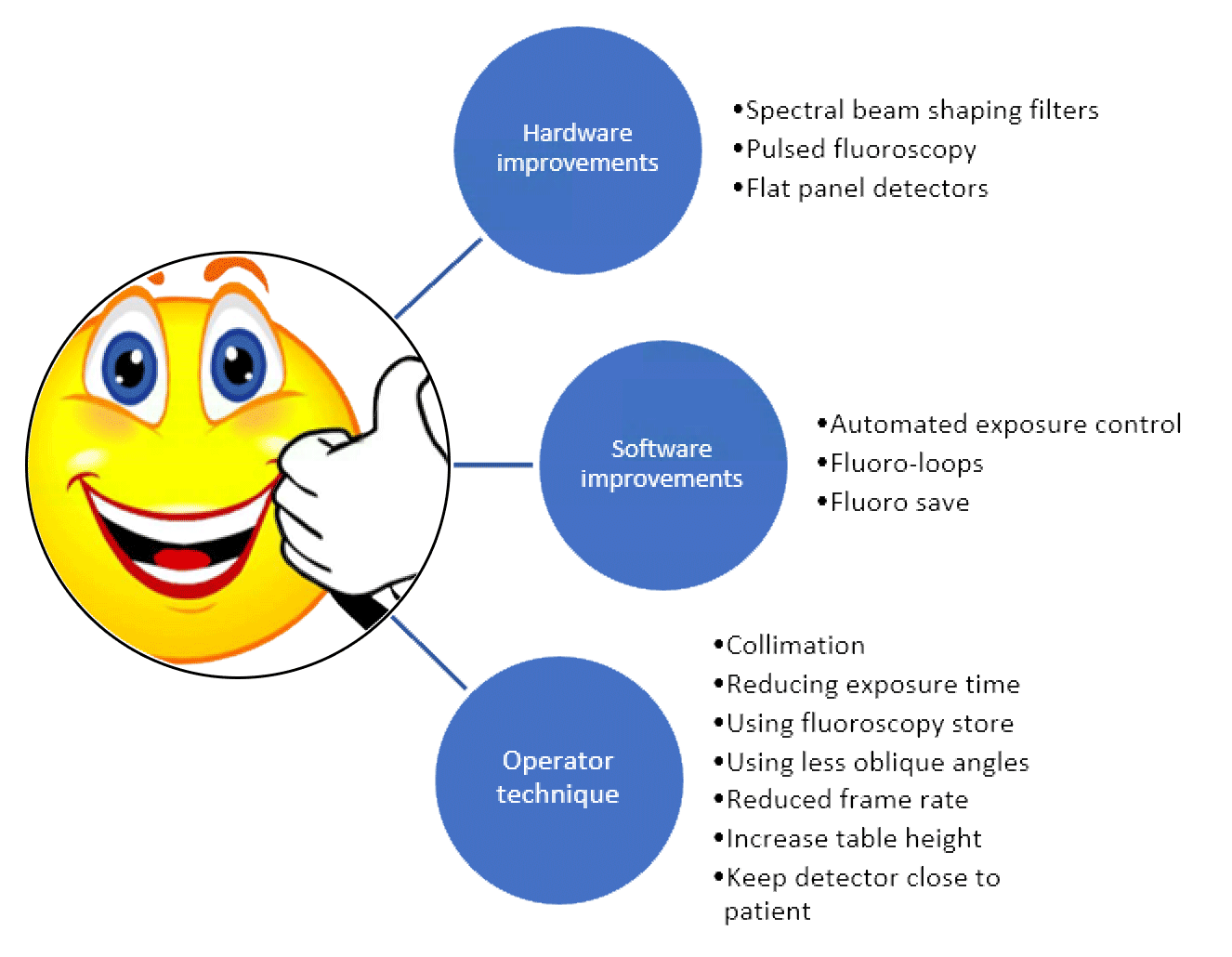
Figure 2. Strategies to reduce the dose to the patient (adapted from 6,8,9)
Another simple way of reducing the dose is by reducing the imaging time or the field of view using collimation. Furthermore a reduction in the frame rate will reduce the dose but will also reduce the temporal resolution. Whilst all of these strategies will help reduce the dose received by the patient, it is important to balance this against the need to ensure the images are of sufficient quality to facilitate the procedure. The addition of fluoroscopy store allows clinicians to review fluoroscopy images rather than using cineangiography, which offers a 90% radiation saving.5 Furthermore, the operator should avoid trying to analyse the image whilst acquiring as this can lead to longer acquisitions. A better approach is to acquire for the shortest possible time to allow diagnosis and then review the acquisition afterwards when no radiation is being used. The introduction of real time radiation dose feedback during procedures can help foster good habits.5,8
Staff perspective
Risks
Similarly to patients it is important to consider both the deterministic and stochastic effects of ionising radiation to staff. Cataract formation commonly results from exposure and has been documented in up to 50% of interventional cardiologists.10 This is a deterministic effect and importantly the dose found to precipitate this has recently been reduced by a factor of 10.4 Skin and hair changes are often found as a deterministic effect in interventional cardiologists.11 Vascular disease (macrovascular and microvascular) has been reported as a complication of radiation therapy, however the occupational significance remains unclear.5,11,12
The main concern for clinical staff is the risk of neoplastic disease which is stochastic in nature. Studying this risk is difficult due to the long latency period which makes it difficult to attribute causation and also the challenge in identifying the additional risk attributable to the radiation exposure over and above the background dose.9 A number of epidemiological studies of clinicians working with ionising radiation in the modern era have found that the risk of neoplastic disease and mortality are similar when compared with clinicians who never worked with radiation.4 However a number of large cohorts that have been actively followed (for a minimum of twenty years and others until the whole cohort had died) up for the development of neoplastic disease have consistently shown that clinicians who started working in the early part of last century did have an excess risk, particularly of developing leukaemia.4 A more recent study of the use of fluoroscopy found an increase in breast cancer, brain cancer and melanoma but it is difficult to exclude confounding when interpreting these data.8 Overall these studies are discrepant as to whether occupational radiation exposure is associated with an increase in neoplastic disease or all-cause mortality.4,8 The evidence for brain tumours resulting from exposure to fluoroscopic procedures is not yet conclusive and mainly circumstantial. An interesting observation from one case series of 26 cases found that 85% of brain tumours were left sided.13 This is not a feature seen in the general population and given the set up in most cardiac catheterisation laboratories (where for the majority of procedures, the operator stands on the patients right with the image intensifier to the left) is suggestive of an association between occupational exposure and the risk of brain tumours.5,12 The thyroid gland has been shown to develop structural and functional changes as a result of radiation and data show that the rate of neoplastic disease increases in proportion to the dose received.5 A number of studies have suggested that there may be an increased risk of breast cancer in female radiation workers and, similarly with brain tumours, these are more common on the left in cardiac catheterisation laboratory workers.5
How to reduce the dose
The majority of the dose that staff receive is scattered from the patient and therefore any strategies that reduce the dose to the patient, as already discussed, will also reduce the dose that staff are exposed to. Table 3 highlights two additional strategies that staff can use to reduce their dose; shielding and increasing the distance from the beam.5,8 Increasing the distance from the source is a powerful way of reducing the dose as the dose follows an inverse square relationship with the distance (i.e. doubling the distance from source will reduce the dose by a factor of four).8 Clearly avoiding the direct beam will prevent the operator from receiving a relatively large dose to the exposed area. The use of a 0.35mm lead equivalent apron will prevent transmission of 96.9% of radiation.8 Thicker lead equivalents will further reduce the received dose but this has to be balanced against the risk of orthopaedic injuries, which have been reported in up to 49% of interventional operators.5 It is important that aprons are well fitted without gaps at the side, particularly as the left side is the most exposed area in standard cardiac catheterisation laboratory set ups. Given the specific risks already discussed, thyroid shields and protective glasses should be worn. Furthermore given the data, radiation protection hats should also be considered. The advantage of table and ceiling mounted shielding is that they do not come with the risk of orthopaedic injuries that protective garments carry but can reduce the dose received by 50%.5 In addition the use of disposable radiation pads, that are placed by the patients side during the procedure can also reduce scattered radiation by up to 95%.5
Table 3. Strategies to reduce staff dose using shielding and distance strategies (adapted from 5,8) | |
|---|---|
Shielding | Distance |
Protective garments including whole body aprons and thyroid shields | Avoid direct exposure to the beam |
Protective hats | Increase the distance from the radiation source |
Protective glasses | Robotic catheter lab |
Table and ceiling mounted mobile shielding | |
Patient radiation pads | |
Monitoring staff dose
The Ionising Radiation (Medical Exposure) Regulations (IR(ME)R) are a set of regulations, enforced by the Care Quality Commission, designed to ensure that ionising radiation is used safely and to protect both patients and staff from the risk of harm.14 This sets out the standards of monitoring for staff exposure. As such, depending on the clinical role, eye, body and finger dosimeters are used to track the exposure of each member of staff. Whilst these do not reduce the dose received, they allow departments to ensure that staff are not being over exposed and to identify any need for adjustments in the working environment.
An historic issue or a modern concern?
Recent improvements in software and hardware have reduced the dose received to staff and patients. This is reflected in the data demonstrating that clinicians practicing more recently have lower rates of developing radiation related complications than historical cohorts. However, the development of new technologies and new techniques (such as chronic total occlusion angioplasty and cardiac resynchronisation pacing), some of which require long and complex procedures and as such are associated with higher radiation doses is one reason why fluoroscopy users need to remain diligent in the 21st century. Furthermore the increasing numbers of patients with obesity, in whom the dose required to maintain sufficient image quality is much higher, again highlights the importance of this issue to all fluoroscopy users. That said, developing techniques such as three-dimensional electrophysiology mapping systems, intracardiac echocardiography and intracoronary imaging have the potential to ameliorate some of the risks.
Conclusion
Despite the technological advances in the use of radiation in the cardiac catheterisation laboratory, there is still a clear risk associated with its use to both patients and the clinical team. Reducing the dose utilised in procedures should be a core skill for all cardiologists and there are a range of simple strategies that can reduce the risk to both staff and patients.
Disclosures
None.
References
- Oatway WB, Jones AL, Holmes S et al. Ionising radiation exposure of the UK population: 2010 review 2016. Public Health England. Available from: https://www.gov.uk/government/publications/ionising-radiation-exposure-of-the-uk-population-2010-review Accessed 16.5.2020.
- Public Health England Guidance. Patient dose information: guidance 2008 Available from: https://www.gov.uk/government/publications/medical-radiation-patient-doses/patient-dose-information-guidance Accessed 16.5.2020
- Picano E, Vano E. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound. 2011;9:35.
- Linet MS, Kim KP, Miller DL, Kleinerman RA, Simon SL, Berrington de Gonzalez A. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat Res. 2010;174(6):793-808.
- Kumar G, Rab S. Radiation Safety for the Interventional Cardiologist—A Practical Approach to Protecting Ourselves From the Dangers of Ionizing Radiation: American College of Cardiology; 2016. Available from: https://www.acc.org/latest-in-cardiology/articles/2015/12/31/10/12/radiation-safety-for-the-interventional-cardiologist Accessed 16.5.20.
- Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V, et al. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J. 2014;35(10):665-72.
- Berrington de Gonzalez A, Kim KP, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the United States. Circulation. 2010;122(23):2403-10.
- Williams MC, Stewart C, Weir NW, Newby DE. Using radiation safely in cardiology: what imagers need to know. Heart. 2019;105(10):798-806.
- Abbott JD. Controlling radiation exposure in interventional cardiology. Circ Cardiovasc Interv. 2014;7(4):425-8.
- Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ. Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76(6):826-34.
- Sun Z, AbAziz A, Yusof AK. Radiation-induced noncancer risks in interventional cardiology: optimisation of procedures and staff and patient dose reduction. Biomed Res Int. 2013;2013:976962.
- Picano E, Vano E, Domenici L, Bottai M, Thierry-Chef I. Cancer and non-cancer brain and eye effects of chronic low-dose ionizing radiation exposure. BMC Cancer. 2012;12:157.
- Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111(9):1368-72.
- Ionising Radiation (Medical Exposure) Regulations (IR(ME)R). Care Quality Commission; 2020. Available at https://www.cqc.org.uk/guidance-providers/ionising-radiation/ionising-radiation-medical-exposure-regulations-irmer Accessed 16.5.2020.
Community Events Calendar


