COVID-19: Implications for Cardiologists
| Take Home Messages |
|---|
|
Introduction
The novel Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) was declared an official pandemic by the World Health Organization (WHO) on the 11th March 2020.1
Patients with COVID-19 and pre-existing cardiovascular diseases have an increased risk of severe disease, characterised by increased need for intensive care support, and death.2-9 Infection has been associated with multiple direct and indirect cardiovascular complications including acute myocardial injury, myocarditis, heart failure, arrhythmias and venous thromboembolism.5,6,10 Furthermore, therapies under investigation for treating COVID-19 patients have potential cardiovascular side effects.6 It is vital therefore that cardiologists have an understanding of the disease and how it affects patients, particularly those with cardiovascular disease who are vulnerable to severe complications and death.
This editorial briefly outlines the background to the COVID-19 pandemic; highlights its clinical presentation; reviews the investigations used to confirm the diagnosis; clarifies the cardiovascular complications and the role of angiotensin converting enzyme-2 (ACE2); and examines the potential therapeutic implications and evolving treatment options. This is a rapidly evolving field and most data is limited to case series and observational data, mainly from China and Italy.
COVID-19 pandemic
SARS-CoV-2 originated in bats and was transmitted to humans through yet unknown intermediary animals in Wuhan, Hubei province, Central China in December 2019.11 As of the 6th April 2020, according to the WHO there have been 1,214,466 cases of COVID-19 reported worldwide with 67,767 related deaths.12 As of 6th April 2020, 51,608 people have tested positive for COVID-19 within the United Kingdom (UK) with 5373 related deaths13 although the actual number of positive cases is expected to be much higher and has been estimated, as of 28th March 2020, to be 2.7% (confidence interval (CI) 1.2-5.4%) of the UK population.14 Notably the death rate of COVID-19 is much higher compared to seasonal influenza that has a mortality rate of around 0.1%. WHO estimates that around 650,000 people die of influenza related respiratory illness annually worldwide.15 A recent article, which used WHO data on cumulative number of deaths to March 2020, suggested that mortality rates would be 5.6% (95% CI 5.4-5.8) in China and 15.2% (12.5-17.9) outside of China.16 While other zoonotic coronaviruses have a higher case fatality rate, Severe Acute Respiratory Syndrome (SARS) 9.6% and Middle East Respiratory Syndrome (MERS-CoV) 34.4%, COVID-19 has already resulted in more deaths than both outbreaks combined.17 This probably relates to its higher infectivity rate. It is estimated that the R0 value (the basic reproduction number which represents viral infectivity) of COVID-19 is 2.28 to compared to SARS (1.88) and MERS-CoV (0.47).18
Clinical presentation and investigations
Just like any other illness, the clinical presentation of COVID-19 is quite variable. The clinical characteristics of mild disease include symptoms common to other viral infections (i.e. fever, cough, myalgia, dyspnoea, diarrhoea and fatigue). A recent study that sampled 1099 laboratory confirmed cases found that the common clinical manifestations included fever (88.7%), cough (67.8%), fatigue (38.1%), sputum production (33.4%), shortness of breath (18.6%), sore throat (13.9%) and headache (13.6%). There were some additional gastrointestinal symptoms observed including diarrhoea (3.8%) and vomiting (5%).8 Table 1 indicates common and uncommon symptoms observed in COVID-19. Approximately 1.5 million people used the recent COVID symptom tracker application, developed by researchers from Kings College London of which 26% reported one or more symptoms. Of these, 1702 reported having been tested for COVID-19, with 579 positive and 1,123 negative results. Subsequent analysis has shown that 59% of COVID-19 positive patients reported loss of smell and taste, compared to 18% of those who tested negative for the disease. The reported loss of smell and taste was shown to be a more reliable indicator of infection with COVID-19, compared to fever.19
A large study from the Chinese Centre for Disease Control and Prevention indicates that clinical severity of COVID-19 was reported as being mild in 81.4%, severe in 13.9% and critical in 4.7%.7 Laboratory investigations often show leukopenia and elevated inflammatory markers including CRP. In severe cases, the clinical course can be complicated by acute respiratory distress syndrome (ARDS), sepsis and septic shock and multi-organ failure including acute kidney injury and cardiac injury.20
Radiographic features
The most common patterns on chest x-ray are unilateral or bilateral lung infiltrates and computed tomography (CT) of the chest often shows ground glass opacity (56.4%) and bilateral patchy shadowing (51.8%).18 A study of 1014 patients who received both RT (reverse transcriptase)-PCR and CT in Wuhan found that 97% of cases with RTPCR confirmed diagnoses had CT findings of pneumonia and concluded that, “CT imaging has high sensitivity for diagnosis of COVID-19”.9 However CT scans of 112 cases with RT-PCR confirmation from the Diamond Princess Cruise showed that less than two-thirds of cases (61%) had lung opacities on CT and 20% of symptomatic cases had negative CT scans.21 A recent correspondence article in the Lancet argued that CT does not add diagnostic value and that positive results can only be believed if the pre-test probability of the disease is high. The feeling from authors was that framing CT as pivotal amidst the COVID-19 pandemic could overwhelm resources and was possibly dangerous and could increase the risk of infection to users as CT scanners could become vectors of infection.22 Table 1 indicates typical and atypical radiological features observed in COVID-19. Figure 1 shows an example of the typical evolving CT chest findings in a 77-year old patient infected with COVID-19.
There is evidence to suggest the important role lung ultrasound (US) can play in the diagnosis of COVID-19.23-25 Table 1 shows the characteristic ultrasound patterns observed in COVID-19 patients.26 Lung US has the benefits of being available at the bedside as a portable test; it reduces risk of potential infection to radiology departments and equipment, is reproducible and has no risk of radiation. However, it is user dependent and relies heavily on user experience. A recent correspondence published in the Lancet suggested that lung US should replace the stethoscope, reducing the risk of cross infection to medical staff.27
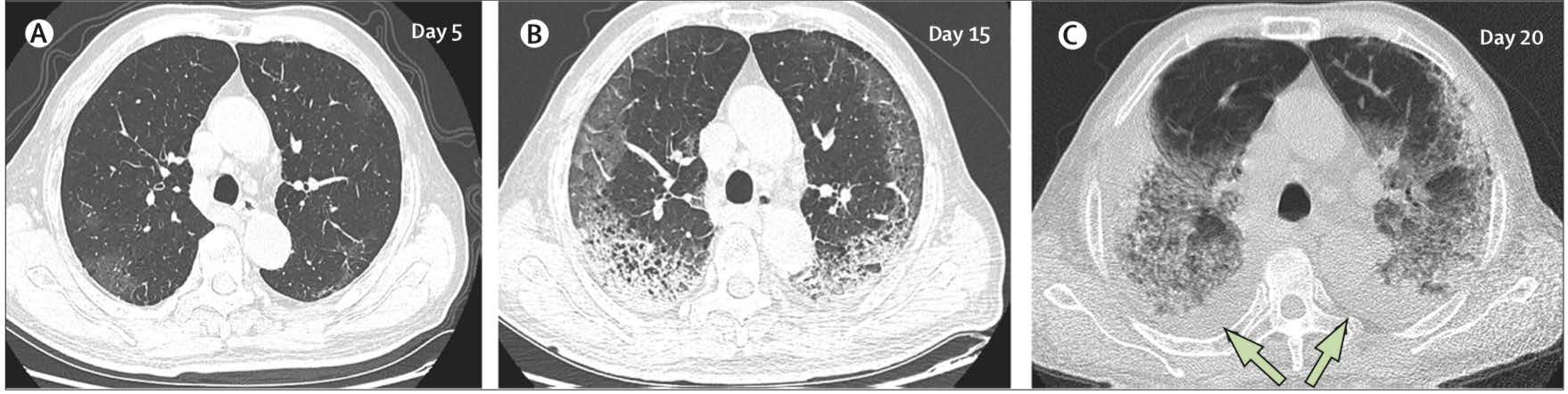 Figure 1: CT chest findings in COVID-19 Transverse thin-section serial CT scans from a 77-year old man (A) Day 5 after symptom onset: patchy ground-glass opacities affecting the bilateral, subpleural lung parenchyma. (B) Day 15: subpleural crescent shaped ground glass opacities in both lungs, as well as posterior reticular opacities and subpleural crescent-shaped consolidations. (C) Day 20: expansion of bilateral pulmonary lesions, with enlargement and denser pulmonary consolidations and bilateral pleural effusions (arrows). The patient died 10 days after the final scan. This file is licensed under the Creative Commons Attribution 4.0 international license. Adapted from Shi H, Han X, Jiang N et al28 CT computed tomography. |
| Table 1. Symptoms, risk factors and tests available for COVID-19 | |
|---|---|
| Symptoms | |
| Common | Fever, Cough, Dyspnea, Anosmia/Dysgeusia, Fatigue, Myalgia, Anorexia, Sputum production, Sore throat |
| Uncommon | Conjunctivitis, Confusion, Dizziness, Headache, GI symptoms, Haemoptysis, Rhinorrhea, Chest pain |
| Rare | Cutaneous manifestations |
| Risk Factors | |
| Residence in/travel to location reporting community transmission during the 14 days prior to symptom onset | |
| Close contact with a confirmed case | |
| Older age/underlying health conditions | |
| Laboratory Tests | |
| Viral PCR | Detection of SARS-CoV-2 Viral RNA Routinely collected from nasopharyngeal swab Can also be collected from endotracheal aspirate or bronchiolar lavage (in intubated patients) |
| Serological Testing | Not available as of yet, but assays in development. Positive for SARS-CoV-2 virus antibodies |
| Radiological Features | |
| Chest x-ray | Unilateral or bilateral lung infiltrates. |
| CT chest | Typical Features: Multiple bilateral lobular and subsegmental areas of groundglass opacity or consolidationa, crazy-paving pattern, air bronchograms, reverse halo/perilobular pattern. Atypical Features: interlobular or septal thickening (smooth or irregular), thickening of the adjacent pleura, subpleural involvement, pleural effusion, pericardial effusion, bronchiectasis, cavitation, pneumothorax, lymphadenopathy, round cystic changes. |
| Lung Ultrasound | B-lines, white lung, pleural line thickening, consolidations with air bronchograms |
| aUsually peripheral or posterior, mainly in the lower lobes, less frequently in right lower lobe. CT computed tomography, PCR polymerase chain reaction, RNA ribose nucleic acid. | |
Diagnostic criteria
The WHO has updated their definitions of case definitions for suspected cases, probable cases and confirmed cases of COVID-19 (See Box 1).29 It is vitally important that all doctors ensure they are wearing the right personal protective equipment (PPE) before approaching a suspected or confirmed case of COVID-19. PPE guidance is changing regularly, so one needs to ensure they are up to date with the latest national and local hospital guidance.30
| Box 1. WHO diagnostic criteria for COVID-19 |
|---|
Suspected case A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g. cough, shortness of breath), AND a history of travel to or residence in a location reporting community transmission of COVID-19 disease during the 14 days prior to symptom onset. OR A patient with any acute respiratory illness AND having been in contact with a confirmed or probable COVID-19 case in the last 14 days prior to symptom onset; OR A patient with severe acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g. cough, shortness of breath; AND requiring hospitalisation) AND in the absence of an alternative diagnosis that fully explains the clinical presentation. |
Probable case A suspect case for whom testing for the COVID-19 virus is inconclusivea. OR A suspect case for whom testing could not beperformed for any reason. |
Confirmed case A person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms. |
| aA person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms. WHO World Health Organization. |
Comorbidities
Early COVID-19 case reports suggest that patients with certain underlying medical conditions are at a higher risk for complications or mortality.2-4 A meta-analysis of six studies inclusive of 1527 COVID-19 patients examined the prevalence of cardiovascular disease and reported the prevalence of hypertension, cardiac and cerebrovascular disease, and diabetes to be 17.1%, 16.4% and 9.7% respectively.2 To date, age>60 years, male sex and presence of comorbidities are known to be the major risk factors for increased mortality in COVID-19. The presence of cardiac injury, myocarditis and ARDS are other strong and independent factors associated with increased mortality risk.31,32
Myocardial injury, myocarditis and acute coronary syndromes
COVID-19 has been associated with myocarditis and there have been reported cases of severe myocarditis with reduced left ventricular systolic function.33,34 Sporadic autopsy cases have shown infiltration of the myocardium by interstitial mononuclear cells.34 There has also been a report of a 53-year-old lady in Italy with COVID-19 who presented with symptoms typical of myocarditis. This was confirmed on cardiac magnetic resonance imaging showing changes typical of myocarditis.35 Her coronary angiogram showed no evidence of coronary disease. She was treated with dobutamine, antiviral drugs (lopinavir/ritonavir), steroids, chloroquine and standard medical treatment for heart failure, which resulted in progressive clinical stabilisation. In a case series of 150 patients with COVID-19, there were 68 deaths of which 7% were directly attributed to myocarditis with circulatory failure with a further 33% in which myocarditis was thought to have played a role in their subsequent death.31
Myocardial injury, defined by an increase in troponin levels, can occur as a result of supply-demand mismatch (i.e. type 2 myocardial infarction) or non-ischemic myocardial pathologies, such as myocarditis. In COVID-19 a number of patients develop myocardial injury in the context of ARDS and hypoxia and there has been evidence suggesting that troponin levels correlate with disease severity.2-4,20,31,34 Furthermore, patients with myocardial injury had a higher incidence of ARDS (58.5% vs 14.7%; p<0.001) and a higher mortality rate (51.2% vs 4.5%; p<0.001) than those without cardiac injury. In subsequent analysis, cardiac injury and ARDS were significantly and independently associated with high mortality, with hazard ratios of 4.26 and 7.89, respectively.36
Although the incidence of acute coronary syndromes (ACS) in COVID-19 has not been well established, it is likely that the inflammatory processes and hemodynamic changes associated with the illness will confer a higher risk of subsequent plaque rupture in susceptible patients.6 Additionally, one should remain vigilant to the potential overlapping clinical presentations of ACS and COVID-19.
Cardiac arrhythmia, shock and cardiac arrest
Hypotension, tachycardia, bradycardia, arrhythmia or even sudden cardiac death are common in patients with SARS.37 Electrocardiographic changes and troponin elevation may signal underlying myocarditis. In a case series of COVID-19 from Wuhan, acute cardiac injury, shock and arrhythmia were present in 7.2%, 8.7% and 16.7% of patients respectively, with preferentially higher prevalence amongst patients requiring intensive care.5
Cardiomyopathy and heart failure
Zhou et al reported that 23% of patients with confirmed COVID-19 infection had evidence of heart failure.4 Patients with heart failure were less likely to survive hospitalisation compared to those that did not (51.9% vs 11.7%).4 It is worth noting that in this series, it is unclear whether the heart failure observed was an exacerbation of pre-existing left ventricular systolic dysfunction, a new cardiomyopathy secondary to myocarditis or a combination of both factors. In SARS, echocardiography frequently demonstrates subclinical left ventricular diastolic impairment, with a higher likelihood of the need for mechanical ventilation in those patients with systolic impairment and reduced ejection fraction.38
ACE2 and potential therapeutic implications
Studies have demonstrated that SARS-CoV2 as well as other coronaviruses use the ACE2 protein for cell entry. ACE2 is a type 1 integral membrane protein that is highly expressed in lung alveolar cells providing the main entry site for the virus into human hosts. ACE2 also serves a role in lung protection and therefore viral binding to this receptor deregulates a lung protective pathway, contributing to viral pathogenicity.6 The complications stemming from modulation of this receptor are not fully understood and are due to be tested in upcoming clinical trials. Despite some literature suggesting the possible harmful effects of ACE inhibitors and angiotensin receptor blockers (ARB) in the setting of COVID-19, The American College of Cardiology, American Heart Association, British Cardiovascular Society, the British Society for Heart Failure and the Council on Hypertension of the European Society of Cardiology all recommend that patients should continue their ACE and ARB inhibitors unless directly advised to stop by a medical practitioner.39-41 Subsequently, an article has been recently published in the New England Journal of Medicine suggesting that abrupt withdrawal of RAAS (Renin-Angiotensin-Aldosterone-System) inhibitors in high-risk patients, including those with heart failure, or previous myocardial infarction, may result in clinical instability and adverse health outcomes. The authors confirm that insufficient data is available to determine the effects of RAAS inhibitors in COVID-19, and proposed an alternative hypothesis suggesting that ACE may be beneficial rather than harmful in patients with lung injury.42
Antiviral therapies and other treatment options
The mainstay of treatment remains supportive care and treatment of complications. To date, no approved preventative vaccines or approved therapies are available for COVID-19, although several are being actively studied. Remdesivir is currently being trialled in moderate and moderate to severe COVID-19 infection. Chloroquine, used primarily as an antimalarial agent, has also been demonstrated to have an inhibitory activity in SARS-CoV2 in vitro.43 In addition, a number of immune modulating drugs are being investigated. These drugs all have potential cardiac side effects namely cardiac toxicity and QT prolonging effects with chloroquine. The US Food and Drug Administration have approved the use of plasma from recovered patients to treat patients with COVID-19 who are critically ill.44
Conclusion
COVID-19 is a rapidly evolving pandemic with significant unknowns. It is clear that patients with underlying cardiovascular conditions have a higher risk of severe infection and death. This infection causes both direct and indirect cardiovascular effects as described in the literature but there may be additional longer-term sequelae, which have not yet been identified. Cardiologists must do their best to protect vulnerable patients as well as themselves in order for them to be able to provide care to the patients that need them during this difficult time.
Disclosures
None.
References
- World Health Organization (WHO). Available: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic [Accessed March 2020]
- Li B, Yang J, Zhao F et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020. DOI: 10.1007/s00392-020-01626-9.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol.2020. DOI: 10.1038/s41569-020-0360-5
- Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. https://doi.org/10.1016/S0140-6736(20)30566-3
- Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; DOI:10.1001/jama.2020.1585.
- Driggin E, Madhavan M, Bikdeli B et al. Cardiovascular considerations for patients, health care workers and health systems during the coronavirus disease 2019 (COVID-19) pandemic. Journal of the American College of Cardiology. March 2020. Available: https://doi.org/10.1016/j.jacc.2020.03.031 [Accessed March 2020]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. DOI:10.1001/jama.2020.2648
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020.
- Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; published online Feb 26.
- Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497-506.
- GuoYan-Rong, Cao Qing-Dong, Hong Zhong-Zi. The origin transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- an update on the status. Military Medical Research. March 2020. Available: https://mmrjournal.biomedcentral.com/articles/10.1186/s40779-020-00240-0 [Accessed March 2020].
- World Health Organization. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed April 2020]
- Available: https://www.gov.uk/guidance/coronaviruscovid-19-information-for-the-public [Accessed April 2020]
- Flaxman S, Mishra S, Gandy A. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. Imperial College COVID-19 response team. March 2020. Available: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-Europe-estimates-and-NPI-impact-30-03-2020.pdf [Accessed March 2020]
- World Health Organization. Available: http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/seasonal-influenza/burden-of-influenza [Accessed March 2020]
- Baud D, Qi X, Nielson-Saines K et al. Real estimates of mortality following COVID-19 infections. The Lancet. Infectious diseases. March 2020. Available: https://doi.org/10.1016/S1473-3099(20)30195-X [Accessed March 2020]
- Mahase E. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020. Available: https://www.bmj.com/content/368/bmj.m641 [Accessed March 2020]
- Wu JT, Leung K, Leung GM. Now casting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. The Lancet. 2020. Available: https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(20)30260-9.pdf [AccessedMarch 2020]
- Kings College Working Group. Available: https://www.kcl.ac.uk/news/loss-of-smell-and-taste-a-key-symptom-for-covid-19-cases [Accessed March 2020]
- Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. DOI: 10.1016/S2213-2600(20)30079-5
- Inui S, Fukikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19). Radiology: Cardiothoracic Imaging 2020; DOI:10.1148/ryct.2020200110.
- Hope M, Raptis C, Shah A et al. A role for CT in COVID-19? What data really tell us so far. The Lancet. March 2020. https://doi.org/10.1016/S0140-6736(20)30728-5
- BuonsensoD, PianoA, RaffaelliF, et al. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pneumonia: a case report and potential applictions during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776-80.
- SoldatiG, SmargiassiA, InchingoloR, et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. Mar 2020. https://doi.org/10.1002/jum.15284
- SoldatiG, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients; a simple, quantitative, reproducible method. J Ultrasound Med. March 2020. https://doi.org/10.1002/jum.15285
- Beeching N, Fletcher T, Fowler R. Coronavirus disease 2019 (COVID-19). BMJ Best Practice. March 2020. Available online: https://bestpractice.bmj.com/topics/en-gb/3000168 [Accessed April 2020]
- Buonenso D, Pata D, Chairetti A. COVID-19 outbreak: less stethoscope, more ultrasound. The Lancet. https://doi.org/10.1016/S2213-2600(20)30120-X
- Shi H, Han X, Cao Y et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China. A descriptive study. Lancet Infect Dis 2020; 425-34
- World Health Organization. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200
401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2 [AccessedApril 2020] - Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/877728/
T1_Recommended_PPE_for_healthcare_workers_by_secondary_care_clinical_context_poster.pdf [Accessed April 2020] - Ruan Q, Yang K, Wang W et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020. DOI:10.1007/s00134-020-05991-x
- Madjid M, Safavi-Naeini P, Solomon S. Potential affects of coronaviruses on the cardiovascular system- A review. JAMA Cardiology. Available: https://jamanetwork.com/journals/jamacardiology/fullarticle/2763846 [Accessed March 2020]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020:S2213- 2600(20)30076-X
- Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement 1 with coronavirus 2019 (COVID-19) infection. JAMA Cardiol. 2020. DOI:10.1001/ jamacardio.2020.1096
- Inciardi R, Lupi L, Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. Available: https://jamanetwork.com/journals/jamacardiology/fullarticle/2763843 [Accessed March 2020]
- Shi S, Qin M, Shen B, et al. Cardiac injury in patients with corona virus disease 2019. JAMA Cardiol. Published online 2020 DOI:10.1001/jamacardio.2020.0950
- Xiong T-Y, Redwood S, Prendergast B et al. Coronaviruses and the cardiovascular system: acute and long-term implications. European Heart Journal. March 2020. Available: https://doi.org/10.1093/eurheartj/ehaa231. [Accessed March 2020]
- Li SS, Cheng C, Fu C et al. Left ventricular performance in patients with severe acute respiratory syndrome: 30 day
echocardiographic follow up study. Circulation. 2003;108:1798-1803 - Madjid M, Solomon SD, Vardeny O et al. Cardiac implications of coronavirus (COVID-19). Available: https://www.acc.org/latest-in-cardiology/articles/2020/02/13/12/42/acc-clinical-bulletin-focuses-on-cardiac-implications-of-coronavirus-2019-ncov [Accessed March 2020]
- Available: https://www.britishcardiovascularsociety.org/news/ACEi-or-ARB-and-COVID-19 [Accessed March 2020]
- Available: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 [Accessed March 2020]
- Vaduganathan M, Vardeny O, Michel et al. Renin-Angiotensin-Aldosterone System Inhibitors in patients with Covid-19. NEJM. March 2020.
- Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269-271.
- Available: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds [Accessed March 2020]
Community Events Calendar


