Anti-inflammatory drugs - can they be a part of the “conventional” secondary prevention medications in ACS?
| Take Home Messages |
|---|
|
Introduction
Coronary artery disease (CAD) and acute coronary syndromes such as myocardial infarction (MI) are pro-inflammatory conditions, involving release of acute phase reactants, such as C-reactive protein (CRP), interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (see Figure 1).1-4 Accumulating evidence suggests that targeting inflammation may improve outcomes in patients with CAD,3-15 but these are yet to be implemented in routine clinical practice. In this editorial I will briefly outline the role of anti-inflammatory drugs in CAD and review the recently published Colchicine Cardiovascular Outcome Trial (COLCOT).13
Anti-inflammatory drugs in coronary artery disease
In the late 1990’s it was observed that baseline plasma concentration of CRP correlated with the risk of future MI and stroke.5 Albert et al. (2001) studied the effect of statins on CRP levels in the PRINCE trial and found that Pravastatin reduced CRP levels at both 12 and 24 weeks irrespective of its effect on low-density lipoprotein cholesterol.6 Then in 2008, The Justification for the Use of Statins in Prevention: an Interventional Trial Evaluating Rosuvastatin (JUPITER) trial showed that Rosuvastatin significantly reduced the incidence of major cardiovascular events in those with raised levels of high sensitivity-CRP (hs-CRP) even in the absence of hyperlipidaemia.7 However, the median follow up in JUPITER was relatively short (1.9 years), which could have possibly resulted in overestimation of the treatment effect.
The role of anti-inflammatory drugs in the management of cardiovascular disease was subsequently explored in various studies.3-15 The effects of Methotrexate therapy on the physical capacity of patients with ischaemic heart failure (METIS) trial studied the effect of Methotrexate (a commonly used immune modulator) in patients with ischaemic chronic heart failure.8 This was a negative trial. Methotrexate had no significant effect on the 6 minute walk test or on serum CRP levels.8 Low dose Methotrexate was also investigated in the Cardiovascular Inflammation Reduction Trial (CIRT), but it failed to reduce levels of IL-1ß, IL-6 or CRP, and it did not reduce the incidence of cardiovascular events in patients with stable atherosclerosis.9 The CIRT trial had a larger sample size, longer duration of follow up and used higher doses of Methotrexate compared to the METIS trial, but had a similar negative outcome. This raised the possibility that the lack of efficacy may be related to the drug being used.
Subsequently in 2017, the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) studied the use of Canakinumab (a monoclonal antibody that selectively inhibits IL-1ß) in patients with a prior MI.10 It concluded that Canakinumab use resulted in lower rates of cardiovascular events in the treatment group compared to placebo, independent of a lipid lowering effect. However this benefit was at the expense of a slightly higher incidence of fatal infections in the treatment group.
The described studies stimulated further research to find an alternative anti-inflammatory drug with a better safety profile. Hence, Colchicine, an inexpensive but potent anti-inflammatory drug was tested in the Low Dose Colchicine (LoDoCo) trial.11 It is an orally administered drug and has numerous anti-inflammatory properties (e.g. anti-tubulin effects, macrophage inhibition, stimulation of antigen presentation and anti-fibrotic properties).12 In the LoDoCO trial, Colchicine was demonstrated to be effective in prevention of cardiovascular events in patients with clinically stable CAD, when used in conjunction with Aspirin and/or Clopidogrel and statins. However, the design of the study was not favourable in that it was single blinded study with a small sample size. Subsequently, the COLCOT trial was conducted.13
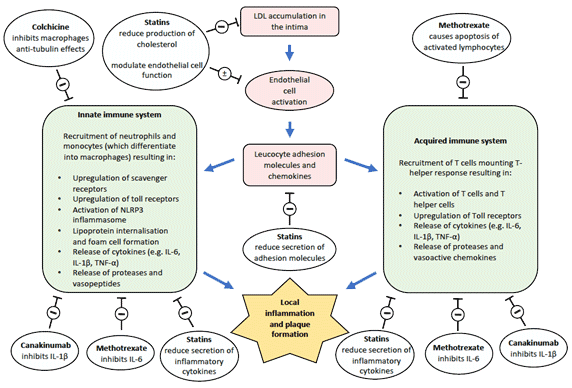
Figure 1. Schematic representation of inflammation in atherosclerosis and therapeutic targets 2-4
Inhibitory (-) or modulatory (±) anti-inflammatory effects of Colchicine, Methotrexate, Canakinumab and statins. IL interleukin, LDL-C low density lipoprotein-cholesterol, NLRP3 NOD-like receptor protein 3, TNF tumor necrosis factor.
The COLCOT trial
Study design
The COLCOT trial was a randomised double blinded multicentre trial, where a total of 4745 patients were enrolled within 30 days after MI, from 167 sites in 12 countries (between December 2015 - August 2018).13 Recruited patients were randomised to either low dose of Colchicine of 0.5 mg per day or placebo. The mean number of days to randomisation was 13.5 days, and the median duration of follow up was 22.6 months.
All patients in the trial had myocardial infarction within 30 days before enrolment, and for which they had been treated according to the contemporary national guidelines (including use of statins). They had all received planned percutaneous revascularisation procedures following the index event (93.0% underwent percutaneous coronary intervention). Aspirin, a different antiplatelet agent and a statin were taken by 98.8%, 97.9% and 99.0% of the patients, respectively. Inclusion and exclusion criteria are included in Table 1.
Table 1. Inclusion and exclusion criteria for the COLCOT trial | |
|---|---|
Inclusion Criteria | Exclusion Criteria |
MI within 30 days before the enrolment | Severe heart failure |
Completed any planned percutaneous revascularization procedure | LVEF less than 35% |
Treated according to national guidelines which included the intensive use of statins | Stroke within the previous 3 months |
Type 2 index MI | |
CABG within the previous 3 years or planned | |
History of non-cutaneous cancer in the previous 3 years | |
Inflammatory bowel disease or chronic diarrhoea | |
Neuromuscular disease or non-transient CK level that was more than 3 times the upper limit of normal (unless due to infarction) | |
Clinically significant non-transient hematologic abnormalities | |
Severe renal disease with a serum creatinine level that was greater than two times the upper limit of the normal range | |
Severe hepatic disease | |
drug or alcohol abuse | |
Current or planned long-term systemic glucocorticoid therapy | |
A history of clinically significant sensitivity to Colchicine | |
CABG coronary artery bypass graft surgery, CK creatinine kinase, LVEF left ventricular ejection fraction, MI Myocardial Infarction. | |
Efficacy and safety outcomes
The primary efficacy end point was a composite of death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalisation for angina leading to coronary revascularisation. Secondary end points were the individual components of the primary efficacy end point.
All serious adverse events were recorded. Other adverse events recorded included: events related to the gastrointestinal system; events attributed to Colchicine or placebo (as judged by the investigators); or clinically significant laboratory abnormalities (as judged by the investigators). Examples of adverse events included diarrhoea, nausea, flatulence, gastrointestinal haemorrhage, anaemia, leukopenia, thrombocytopenia, infection, pneumonia, septic shock, hospitalisation for heart failure, cancer.
Coronary revascularisation, hospitalisation for heart failure, atrial fibrillation, and deep venous thrombosis or pulmonary embolus were additional pre-specified exploratory end points together with the change from baseline to 6 months in the serum hs-CRP level, and the change from baseline to 12 months in the white cell count. An optional CRP biomarker sub-study was conducted in 34 sites and included 207 patients.
Results
The rates of primary outcome (composite of death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalisation for angina leading to coronary revascularisation) were significantly lower in the Colchicine group compared to placebo (5.5% v 7.1%, hazard ratio (HR) 0.77, 95% confidence interval (CI) 0.61-0.96; p=0.02) (see Table 2).
Secondary outcome analysis showed that rates of stroke and urgent hospitalization from angina leading to revascularisation were also less frequent in the Colchicine group compared with the placebo group (see Table 2). There was no significant difference in all cause death, death from cardiovascular causes or myocardial infarction.
There were no significant differences in inflammatory biomarkers between the groups. The adjusted geometric mean percent changes in the hs-CRP level at 6 months after MI were −70.0% and −66.6% in the Colchicine and placebo groups respectively. The placebo-adjusted geometric mean percent change was −10.1% in the Colchicine group (95% CI −28.6 to +13.4). Total white blood cell count measured in a subgroup of patients fell similarly in both trial groups by 1 year, (-18.8% Colchicine, -19.0% placebo).
Diarrhoea occurred similarly in the Colchicine and placebo groups (9.7% v 8.9% respectively; p=0.35). Pneumonia was more common in the Colchicine group compared to the placebo group (0.9% v 0.4%; p=0.03), however the incidence of septic shock was not statistically different between the trial groups, and no deaths from infection were reported.
Table 2. Results of primary and secondary outcomes in the COLCOT Trial | ||||
|---|---|---|---|---|
Primary outcome | Colchicine | Placebo | HR (95% CI) | P value |
Primary efficacy,a % | 5.5 | 7.1 | 0.77 (0.61-0.96) | 0.02 |
Secondary outcomes | ||||
Death from cardiovascular causes, % | 0.8% | 1.0% | 0.84 (0.46-1.52) | - |
MI, % | 3.8 | 4.1 | 0.91 (0.68-1.21) | - |
Stroke, % | 0.2 | 0.8 | 0.26 (0.1-0.7) | - |
Urgent hospitalisation from angina leading to revascularization, % | 1.1% | 2.1% | 0.50 (0.31-0.81) | - |
All cause death, % | 1.8 | 1.8% | 0.98 (0.64-1.49) | - |
aprimary efficacy was a composite of death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalisation for angina leading to coronary revascularisation. CI confidence interval, HR hazard ratio, MI myocardial infarction. | ||||
Discussion
The COLCOT trial showed a favourable outcome for the use of low dose Colchicine (0.5 mg daily) in patients with recent MI. Colchicine significantly reduced the incidence of primary composite end point of death from cardiovascular causes, resuscitated cardiac arrest, recurrent MI, stroke or urgent hospitalisation for angina leading to coronary revascularisation at a median follow up time of 22.6 months. A substudy analysis suggested that the positive outcome was mainly driven by reduction in the risk of urgent hospitalisation for angina leading to revascularisation and reduction in the risk of stroke.
The COLCOT trial was a well conducted and adequately-powered RCT which provides further evidence for the role of anti-inflammatory drugs in treatment of cardiovascular disease. Furthermore, Colchicine is a cheap, widely available drug that is commonly used in clinical practice (i.e. for the treatment of gout and pericarditis). The results of the COLCOT trial support the use of low-dose Colchicine as an adjunct in patients with acute myocardial infarction. However, a number of important limitations should be considered. For example, the use of a composite primary end point may have resulted in overestimation of the effectiveness of the treatment. In addition, there was a higher incidence of pneumonia in the Colchicine group compared to placebo although this did not lead to sepsis or death.
In the subgroup analysis, Colchicine did not reduce death from cardiovascular or all causes, or reduce risk MI, but did reduce risk of urgent hospitalisation for angina leading to revascularisation. Angina/urgent hospitalisation for angina may be considered a soft clinical end point, that is probably at least in part dependent on the assessment of the clinician, and hence may be subjective to bias. In addition, details on the coronary lesions and/or vessels that were revascularised were not mentioned. It was not reported if the treated angina that led to the subsequent admission was caused by a bystander disease that had not been addressed in the prior admission, or by the same lesion that caused the index event (i.e. instent restenosis). The nature of the revascularisation that was ultimately offered (percutaneous coronary intervention or bypass graft surgery) in these subsequent admissions was not mentioned either.
Inflammatory biomarkers were tested in a small subgroup of patients (n=207) who had similar baseline characteristics to the study population. Although serum hs-CRP levels fell by 6 months following MI, there was no significant difference between the Colchicine and Placebo groups. A possible explanation for this finding is that following acute MI, inflammatory markers naturally fall as a reflection of the healing process, and it is possible that Colchicine may quicken this process. In the Value of Colchicine in the treatment of patients with acute myocardial infarction and inflammatory response (COLIN) trial, RCT assessing use of Colchicine in ST-elevation MI, the primary endpoint of peak CRP during index hospitalisation was unaffected by Colchicine.14 Unfortunately, hs-CRP was only measured at baseline and 6 months in the COLCOT trial (and only during index hospitalisation in the COLIN trial), and therefore we cannot rule out subacute effects of Colchicine on inflammation. Drawing firm conclusions regarding the anti-inflammatory effects of Colchicine post MI is difficult given the limitations discussed and the small number of non-randomised patients included in the analysis.
The precise mechanisms by which Colchicine resulted in lower stroke incidence but not MI is not clear from this study, given that both are characterised by acute plaque rupture. Possible explanations include differential effects of Colchicine on different vascular beds; positive effect of Colchicine on cardiac remodelling reducing risk of left ventricular thrombus formation and cardio-embolism15; and reduction in incidence of atrial fibrillation.16 Further work is required to improve our understanding of the cardiovascular effects of Colchicine.
Conclusion
Among patients with a recent MI, low dose of Colchicine of 0.5 mg daily significantly lowered the risk of ischaemic cardiovascular events compared to placebo. The benefit of Colchicine post MI was mainly driven by reduction in the risk of stroke and urgent hospitalisation for angina. Large randomised controlled trials with longer duration of follow up are needed to confirm these findings; establish the correct dosing and duration of Colchicine treatment in patient with recent MI; and highlight the mechanisms by which Colchicine reduces risk of cardiovascular events and stroke in these patients.
Disclosures
None.
References
- Moreira DM, da Silva RL, Vieira JL, et al. Role of vascular inflammation in coronary artery disease: potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. Am J Cardiovasc Drugs 2015 Feb;15(1):1-11. doi: 10.1007/ s40256-014-0094-z.
- Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol 2006; 1:297-329.
- Vaidya K, Martínez G, Patel S. The Role of Colchicine in Acute Coronary Syndrome. Clin Ther 2019;41(1):11-20.
- Balanescu A, Bojinca V, Bojinca M, et al. Cardiovascular effects of methotrexate in immune-mediated inflammatory diseases. Exp Ther Med 2019 Feb; 17(2): 1024–9.
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973-9 [Erratum, N Engl J Med 1997;337:356.].
- Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels. The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001;286(1):64-70. doi:10.1001/jama.286.1.64.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-2207. doi: 10.1056/NEJMoa0807646.
- Moreira DM, Viera JL, Gottschall CA. The effects of METhotrexate therapy on the physical capacity of patients with ISchemic heart failure: a randomized double-blind, placebo-controlled trial (METIS trial). J Card Fail 2009 Dec;15(10):828-34. doi: 10.1016/j.cardfail.2009.06.439.
- Ridker PM, Everett BM, Pradhan A. Low-Dose Methotrexate for The Prevention of Atherosclerotic Events. N Engl J Med 2019; 380:752-62. DOI: 10.1056/NEJMoa1809798.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119-31. doi: 10.1056/NEJMoa1707914.
- Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61: 404-10.
- Leung YY, Yao Hui LL, Kraus VB. Colchicine ---Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015 Dec;45(3):341-50. DOI: 10.1016/j.semarthrit.2015.06.013.
- Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019; 381:2497-2505. doi: 10.1056/NEJMoa1912388.
- Akodad M, Lattuca B, Nagot N, et al. COLIN trial: Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis 2017 Jun - Jul;110(6-7):395-402. doi: 10.1016/j.acvd.2016.10.004.
- Fujisue K, Sugamura K, Kurokawa H, et al. Colchicine Improves Survival, Left Ventricular Remodeling, and Chronic Cardiac Function After Acute Myocardial Infarction. Circ J 2017 Jul 25;81(8):1174-82. doi: 10.1253/circj.CJ-16-0949.
- Harada M, Van Wagoner D, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J 2015; 79(3): 495–502. doi: 10.1253/circj.CJ-15-0138.
- Imazio M, Brucato A, Ferrazzi P, et al. Colchicine Reduces Postoperative Atrial Fibrillation. Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) Atrial Fibrillation Substudy. Circ 2011;124(21): 2290-5.
Community Events Calendar


